Selection Effects and COVID-19 Mortality Risk after Pfizer vs. Moderna Vaccination: Evidence from Linked Mortality and Vaccination Records
- PMID: 37243075
- PMCID: PMC10221876
- DOI: 10.3390/vaccines11050971
Selection Effects and COVID-19 Mortality Risk after Pfizer vs. Moderna Vaccination: Evidence from Linked Mortality and Vaccination Records
Abstract
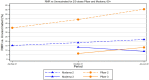
Prior research generally finds that the Pfizer-BioNTech (BNT162b2) and Moderna (mRNA1273) COVID-19 vaccines provide similar protection against mortality, sometimes with a Moderna advantage due to slower waning. However, most comparisons do not address selection effects for those who are vaccinated and with which vaccine. We report evidence on large selection effects, and use a novel method to control for these effects. Instead of directly studying COVID-19 mortality, we study the COVID-19 excess mortality percentage (CEMP), defined as the COVID-19 deaths divided by non-COVID-19 natural deaths for the same population, converted to a percentage. The CEMP measure uses non-COVID-19 natural deaths to proxy for population health and control for selection effects. We report the relative mortality risk (RMR) for each vaccine relative to the unvaccinated population and to the other vaccine, using linked mortality and vaccination records for all adults in Milwaukee County, Wisconsin, from 1 April 2021 through 30 June 2022. For two-dose vaccinees aged 60+, RMRs for Pfizer vaccinees were consistently over twice those for Moderna, and averaged 248% of Moderna (95% CI = 175%,353%). In the Omicron period, Pfizer RMR was 57% versus 23% for Moderna. Both vaccines demonstrated waning of two-dose effectiveness over time, especially for ages 60+. For booster recipients, the Pfizer-Moderna gap is much smaller and statistically insignificant. A possible explanation for the Moderna advantage for older persons is the higher Moderna dose of 100 μg, versus 30 μg for Pfizer. Younger persons (aged 18-59) were well-protected against death by two doses of either vaccine, and highly protected by three doses (no deaths among over 100,000 vaccinees). These results support the importance of a booster dose for ages 60+, especially for Pfizer recipients. They suggest, but do not prove, that a larger vaccine dose may be appropriate for older persons than for younger persons.
Keywords: BNT162b2; COVID-19 excess mortality percentage; COVID-19 mortality rates; Moderna vaccine; Pfizer vaccine; Pfizer-BioNTech vaccine; mRNA1273; vaccine effectiveness.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Understanding COVID-19 Vaccine Effectiveness Against Death Using a Novel Measure: COVID Excess Mortality Percentage.Res Sq [Preprint]. 2022 Dec 16:rs.3.rs-2359020. doi: 10.21203/rs.3.rs-2359020/v1. Res Sq. 2022. Update in: Vaccines (Basel). 2023 Feb 07;11(2):379. doi: 10.3390/vaccines11020379. PMID: 36561183 Free PMC article. Updated. Preprint.
-
Understanding COVID-19 Vaccine Effectiveness against Death Using a Novel Measure: COVID Excess Mortality Percentage.Vaccines (Basel). 2023 Feb 7;11(2):379. doi: 10.3390/vaccines11020379. Vaccines (Basel). 2023. PMID: 36851256 Free PMC article.
-
Comparative Effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) Vaccines in Preventing COVID-19 Hospitalizations Among Adults Without Immunocompromising Conditions - United States, March-August 2021.MMWR Morb Mortal Wkly Rep. 2021 Sep 24;70(38):1337-1343. doi: 10.15585/mmwr.mm7038e1. MMWR Morb Mortal Wkly Rep. 2021. PMID: 34555004 Free PMC article.
-
COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines.Eur Rev Med Pharmacol Sci. 2021 Feb;25(3):1663-1669. doi: 10.26355/eurrev_202102_24877. Eur Rev Med Pharmacol Sci. 2021. PMID: 33629336 Review.
-
The Vaccine Efficacy Against the SARS-CoV-2 Omicron: A Systemic Review and Meta-Analysis.Front Public Health. 2022 Jul 13;10:940956. doi: 10.3389/fpubh.2022.940956. eCollection 2022. Front Public Health. 2022. PMID: 35910897 Free PMC article.
Cited by
-
Vaccinating against a Novel Pathogen: A Critical Review of COVID-19 Vaccine Effectiveness Evidence.Microorganisms. 2023 Dec 31;12(1):89. doi: 10.3390/microorganisms12010089. Microorganisms. 2023. PMID: 38257917 Free PMC article. Review.
-
Evidence on COVID-19 Mortality and Disparities Using a Novel Measure, COVID excess mortality percentage: Evidence from Indiana, Wisconsin, and Illinois.PLoS One. 2024 Jan 31;19(1):e0295936. doi: 10.1371/journal.pone.0295936. eCollection 2024. PLoS One. 2024. PMID: 38295114 Free PMC article.
-
COVID-19 Boosters: If The US Had Matched Israel's Speed And Take-Up, An Estimated 29,000 US Lives Would Have Been Saved.Health Aff (Millwood). 2023 Dec;42(12):1747-1757. doi: 10.1377/hlthaff.2023.00718. Health Aff (Millwood). 2023. PMID: 38048511 Free PMC article.
-
Public health emergency accelerated research response-the Clinical and Translational Science Institute of Southeast Wisconsin COVID-19 research initiative.Front Public Health. 2025 May 9;13:1529121. doi: 10.3389/fpubh.2025.1529121. eCollection 2025. Front Public Health. 2025. PMID: 40416685 Free PMC article.
-
COVID-19 Vaccination Effectiveness in the General Population of an Italian Province: Two Years of Follow-Up.Vaccines (Basel). 2023 Aug 4;11(8):1325. doi: 10.3390/vaccines11081325. Vaccines (Basel). 2023. PMID: 37631893 Free PMC article.
References
-
- European Center for Disease Control Effectiveness and Safety of EU/EEA-authorized Vaccines Against COVID-19: Living Systematic Review. [(accessed on 29 April 2023)]. Available online: https://covid19-vaccines-efficacy.ecdc.europa.eu.
-
- UK Natinal Health Service About COVID-19 Vaccination. [(accessed on 26 April 2023)]. Available online: https://www.nhs.uk/conditions/covid-19/covid-19-vaccination/about-covid-...
-
- US Centers for Disease Control Overview of COVID-19 Vaccination. [(accessed on 26 April 2023)]; Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-co...
-
- Atanasov V., Barreto N., Whittle J., Meurer J., Weston B.W., Luo Q., Franchi L., Yuan A.Y., Zhang R., Black B. Understanding COVID-19 Vaccine Effectiveness against Death Using a Novel Measure: COVID Excess Mortality Percentage. Vaccines. 2023;11:379. doi: 10.3390/vaccines11020379. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

