Mucosal Vaccination with Live Attenuated Bordetella bronchiseptica Protects against Challenge in Wistar Rats
- PMID: 37243086
- PMCID: PMC10224215
- DOI: 10.3390/vaccines11050982
Mucosal Vaccination with Live Attenuated Bordetella bronchiseptica Protects against Challenge in Wistar Rats
Abstract
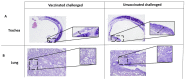
Bordetella bronchiseptica (Bb) is a Gram-negative bacterium responsible for canine infectious respiratory disease complex (CIRDC). Several vaccines targeting this pathogen are currently licensed for use in dogs, but their mechanism of action and the correlates of protection are not fully understood. To investigate this, we used a rat model to examine the immune responses induced and the protection conferred by a canine mucosal vaccine after challenge. Wistar rats were vaccinated orally or intranasally on D0 and D21 with a live attenuated Bb vaccine strain. At D35, the rats of all groups were inoculated with 103 CFU of a pathogenic strain of B. bronchiseptica. Animals vaccinated via either the intranasal or the oral route had Bb-specific IgG and IgM in their serum and Bb-specific IgA in nasal lavages. Bacterial load in the trachea, lung, and nasal lavages was lower in vaccinated animals than in non-vaccinated control animals. Interestingly, coughing improved in the group vaccinated intranasally, but not in the orally vaccinated or control group. These results suggest that mucosal vaccination can induce mucosal immune responses and provide protection against a Bb challenge. This study also highlights the advantages of a rat model as a tool for studying candidate vaccines and routes of administration for dogs.
Keywords: Bordetella bronchiseptica; canine infectious respiratory disease complex (CIRDC); live attenuated vaccines; mucosal vaccination; rat model.
Conflict of interest statement
Among the authors, E.M., G.S., H.P., K.D.L. and L.P. are employees of Boehringer Ingelheim.
Figures





References
LinkOut - more resources
Full Text Sources
Miscellaneous

