Biological Activity of Optimized Codon Bovine Type III Interferon Expressed in Pichia pastoris
- PMID: 37243187
- PMCID: PMC10221290
- DOI: 10.3390/v15051101
Biological Activity of Optimized Codon Bovine Type III Interferon Expressed in Pichia pastoris
Abstract
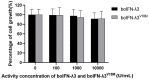
Type III interferons (IFN-λs) exhibit potent antiviral activity and immunomodulatory effects in specific cells. Nucleotide fragments of the bovine ifn-λ (boifn-λ) gene were synthetized after codon optimization. The boifn-λ gene was then amplified by splicing using overlap extension PCR (SOE PCR), resulting in the serendipitous acquisition of the mutated boIFN-λ3V18M. The recombinant plasmid pPICZαA-boIFN-λ3/λ3V18M was constructed, and the corresponding proteins were expressed in Pichia pastoris with a high-level extracellular soluble form. Dominant expression strains of boIFN-λ3/λ3V18M were selected by Western blot and ELISA and cultured on a large scale, and the recombinant proteins purified by ammonium sulfate precipitation and ion exchange chromatography yielded 1.5g/L and 0.3 g/L, with 85% and 92% purity, respectively. The antiviral activity of boIFN-λ3/λ3V18M exceeded 106 U/mg, and they were neutralized with IFN-λ3 polyclonal antibodies, were susceptible to trypsin, and retained stability within defined pH and temperature ranges. Furthermore, boIFN-λ3/λ3V18M exerted antiproliferative effects on MDBK cells without cytotoxicity at 104 U/mL. Overall, boIFN-λ3 and boIFN-λ3V18M did not differ substantially in biological activity, except for reduced glycosylation of the latter. The development of boIFN-λ3 and comparative evaluation with the mutant provide theoretical insights into the antiviral mechanisms of boIFN-λs and provide material for therapeutic development.
Keywords: Pichia pastoris; antiproliferative activity; antiviral activity; bovine interferon–λ3; codon optimization; glycosylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

