Virus-like Particle Vaccines and Platforms for Vaccine Development
- PMID: 37243195
- PMCID: PMC10223759
- DOI: 10.3390/v15051109
Virus-like Particle Vaccines and Platforms for Vaccine Development
Abstract
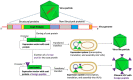
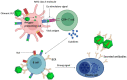
Virus-like particles (VLPs) have gained a lot of interest within the past two decades. The use of VLP-based vaccines to protect against three infectious agents-hepatitis B virus, human papillomavirus, and hepatitis E virus-has been approved; they are very efficacious and offer long-lasting immune responses. Besides these, VLPs from other viral infectious agents (that infect humans, animals, plants, and bacteria) are under development. These VLPs, especially those from human and animal viruses, serve as stand-alone vaccines to protect against viruses from which the VLPs were derived. Additionally, VLPs, including those derived from plant and bacterial viruses, serve as platforms upon which to display foreign peptide antigens from other infectious agents or metabolic diseases such as cancer, i.e., they can be used to develop chimeric VLPs. The goal of chimeric VLPs is to enhance the immunogenicity of foreign peptides displayed on VLPs and not necessarily the platforms. This review provides a summary of VLP vaccines for human and veterinary use that have been approved and those that are under development. Furthermore, this review summarizes chimeric VLP vaccines that have been developed and tested in pre-clinical studies. Finally, the review concludes with a snapshot of the advantages of VLP-based vaccines such as hybrid/mosaic VLPs over conventional vaccine approaches such as live-attenuated and inactivated vaccines.
Keywords: chimeric VLPs; efficacy; immunogenicity; vaccines; virus-like particles.
Conflict of interest statement
Ebenezer Tumban is a co-inventor of an L2-bacteriophage VLP-related patent, managed by the University of New Mexico in accordance with its conflict of interest policies.
Figures
References
-
- Pumpens P., Pushko P. Virus-like Particles: A Comprehensive Guide. CRC Press; Boca Raton, FL, USA: 2022.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

