Efficacy and Durability of Dolutegravir- or Darunavir-Based Regimens in ART-Naïve AIDS- or Late-Presenting HIV-Infected Patients
- PMID: 37243208
- PMCID: PMC10224150
- DOI: 10.3390/v15051123
Efficacy and Durability of Dolutegravir- or Darunavir-Based Regimens in ART-Naïve AIDS- or Late-Presenting HIV-Infected Patients
Abstract
Background: Since limited data are available, we aimed to compare the efficacy and durability of dolutegravir and darunavir in advanced naïve patients.
Methods: Retrospective multicenter study including AIDS- or late-presenting (def. CD4 ≤ 200/µL) HIV-infected patients starting dolutegravir or ritonavir/cobicistat-boosted darunavir+2NRTIs. Patients were followed from the date of first-line therapy initiation (baseline, BL) to the discontinuation of darunavir or dolutegravir, or for a maximum of 36 months of follow-up.
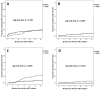
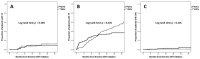
Results: Overall 308 patients (79.2% males, median age 43 years, 40.3% AIDS-presenters, median CD4 66 cells/µL) were enrolled; 181 (58.8%) and 127 (41.2%) were treated with dolutegravir and darunavir, respectively. Incidence of treatment discontinuation (TD), virological failure (VF, defined as a single HIV-RNA > 1000 cp/mL or two consecutive HIV-RNA > 50 cp/mL after 6 months of therapy or after virological suppression had been achieved), treatment failure (the first of TD or VF), and optimal immunological recovery (defined as CD4 ≥ 500/µL + CD4 ≥ 30% + CD4/CD8 ≥ 1) were 21.9, 5.2, 25.6 and 1.4 per 100 person-years of follow-up, respectively, without significant differences between dolutegravir and darunavir (p > 0.05 for all outcomes). However, a higher estimated probability of TD for central nervous system (CNS) toxicity (at 36 months: 11.7% vs. 0%, p = 0.002) was observed for dolutegravir, whereas darunavir showed a higher probability of TD for simplification (at 36 months: 21.3% vs. 5.7%, p = 0.046).
Conclusions: Dolutegravir and darunavir showed similar efficacy in AIDS- and late-presenting patients. A higher risk of TD due to CNS toxicity was observed with dolutegravir, and a higher probability of treatment simplification with darunavir.
Keywords: HIV-1; advanced naïve; antiretroviral therapy; integrase inhibitors; protease inhibitors; treatment discontinuation; virological failure.
Conflict of interest statement
M.F. received speakers’ honoraria, support for travel to meetings and fees for attending advisory boards from Bristol-Myers Squibb (BMS), Gilead, Janssen-Cilag, Merck Sharp & Dohme (MSD), ViiV Healtcare. B.R. received speakers’ honoraria and support for travel to meetings from Bristol-Myers Squibb (BMS), Gilead Sciences, Janssen-Cilag, Merck Sharp & Dohme (MSD), ViiV Healthcare, and fees for attending advisory boards from Gilead Sciences, Janssen-Cilag and ViiV Healthcare. F.L. received fees for attending advisory boards from Janssen-Cilag and ViiV Healthcare, received speakers’ honoraria and support for travel to meetings Gilead, Janssen-Cilag, Merck Sharp & Dohme (MSD), ViiV Healthcare. M.C. received support for travel to meetings from Gilead, JC, ViiV Healthcare, Abbvie and MSD. For the remaining authors none were declared.
Figures

References
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Department of Health and Human Services. [(accessed on 1 April 2021)]; Available online: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/Ad....
-
- EACS Secretariat EACS Guidelines Version 11.0. [(accessed on 22 September 2022)]. Available online: https://www.eacsociety.org/media/final2021eacsguidelinesv11.0_oct2021.pdf.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

