Current Hepatitis C Vaccine Candidates Based on the Induction of Neutralizing Antibodies
- PMID: 37243237
- PMCID: PMC10220683
- DOI: 10.3390/v15051151
Current Hepatitis C Vaccine Candidates Based on the Induction of Neutralizing Antibodies
Abstract
The introduction of direct-acting antivirals (DAAs) has revolutionized hepatitis C treatment. Short courses of treatment with these drugs are highly beneficial to patients, eliminating hepatitis C virus (HCV) without adverse effects. However, this outstanding success is tempered by the continuing difficulty of eradicating the virus worldwide. Thus, access to an effective vaccine against HCV is strongly needed to reduce the burden of the disease and contribute to the elimination of viral hepatitis. The recent failure of a T-cell vaccine based on the use of viral vectors expressing the HCV non-structural protein sequences to prevent chronic hepatitis C in drug users has pointed out that the induction of neutralizing antibodies (NAbs) will be essential in future vaccine candidates. To induce NAbs, vaccines must contain the main target of this type of antibody, the HCV envelope glycoproteins (E1 and E2). In this review, we summarize the structural regions in E1 and E2 proteins that are targeted by NAbs and how these proteins are presented in the vaccine candidates currently under development.
Keywords: envelope glycoproteins; hepatitis C virus; neutralizing antibodies; vaccine development.
Conflict of interest statement
P.R. is co-inventor of the patent “Novel fusion proteins and use thereof for preparing hepatitis C vaccines”; E.G.-E., P.R. and E.B. are co-inventors of the patent “New immunogenic compositions and their use in the preparation of vaccines against hepatitis C and hepatitis B”.
Figures

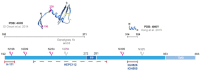
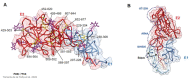
Similar articles
-
A Recombinant Hepatitis C Virus Genotype 1a E1/E2 Envelope Glycoprotein Vaccine Elicits Antibodies That Differentially Neutralize Closely Related 2a Strains through Interactions of the N-Terminal Hypervariable Region 1 of E2 with Scavenger Receptor B1.J Virol. 2019 Oct 29;93(22):e00810-19. doi: 10.1128/JVI.00810-19. Print 2019 Nov 15. J Virol. 2019. PMID: 31462563 Free PMC article.
-
A Novel Approach To Display Structural Proteins of Hepatitis C Virus Quasispecies in Patients Reveals a Key Role of E2 HVR1 in Viral Evolution.J Virol. 2020 Aug 17;94(17):e00622-20. doi: 10.1128/JVI.00622-20. Print 2020 Aug 17. J Virol. 2020. PMID: 32554700 Free PMC article.
-
Virus-Like Particles Containing the E2 Core Domain of Hepatitis C Virus Generate Broadly Neutralizing Antibodies in Guinea Pigs.J Virol. 2022 Mar 9;96(5):e0167521. doi: 10.1128/JVI.01675-21. Epub 2022 Jan 5. J Virol. 2022. PMID: 34986001 Free PMC article.
-
Hepatitis C virus vaccine candidates inducing protective neutralizing antibodies.Expert Rev Vaccines. 2016 Dec;15(12):1535-1544. doi: 10.1080/14760584.2016.1194759. Epub 2016 Jun 13. Expert Rev Vaccines. 2016. PMID: 27267297 Free PMC article. Review.
-
Hepatitis C Virus Envelope Glycoproteins: A Balancing Act of Order and Disorder.Front Immunol. 2018 Aug 24;9:1917. doi: 10.3389/fimmu.2018.01917. eCollection 2018. Front Immunol. 2018. PMID: 30197646 Free PMC article. Review.
Cited by
-
Minicircle-based vaccine induces potent T-cell and antibody responses against hepatitis C virus.Sci Rep. 2024 Nov 4;14(1):26698. doi: 10.1038/s41598-024-78049-3. Sci Rep. 2024. PMID: 39496832 Free PMC article.
-
Systematic collection, annotation, and pattern analysis of viral vaccines in the VIOLIN vaccine knowledgebase.Front Cell Infect Microbiol. 2025 Feb 7;15:1509226. doi: 10.3389/fcimb.2025.1509226. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 39991713 Free PMC article.
-
Future Prospects, Approaches, and the Government's Role in the Development of a Hepatitis C Virus Vaccine.Pathogens. 2023 Dec 31;13(1):38. doi: 10.3390/pathogens13010038. Pathogens. 2023. PMID: 38251345 Free PMC article. Review.
-
Broadly Neutralizing Antibody Characteristics in Hepatitis C Virus Infection and Implications for Vaccine Design.Vaccines (Basel). 2025 Jun 6;13(6):612. doi: 10.3390/vaccines13060612. Vaccines (Basel). 2025. PMID: 40573943 Free PMC article. Review.
-
Virus-Mimicking Polymer Nanocomplexes Co-Assembling HCV E1E2 and Core Proteins with TLR 7/8 Agonist-Synthesis, Characterization, and In Vivo Activity.J Funct Biomater. 2025 Jan 19;16(1):34. doi: 10.3390/jfb16010034. J Funct Biomater. 2025. PMID: 39852590 Free PMC article.
References
-
- World Health Organization . Accelerating Access to Hepatitis C Diagnostics and Treatment. Global Progress Report 2020. World Health Organization; Geneva, Switzerland: 2021.
-
- World Health Organization . Global Hepatitis Report, 2017. World Health Organization; Geneva, Switzerland: 2017.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

