Comparing Heterologous and Homologous COVID-19 Vaccination: A Longitudinal Study of Antibody Decay
- PMID: 37243247
- PMCID: PMC10222288
- DOI: 10.3390/v15051162
Comparing Heterologous and Homologous COVID-19 Vaccination: A Longitudinal Study of Antibody Decay
Abstract
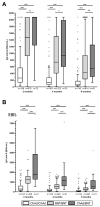
The humoral response after vaccination was evaluated in 1248 individuals who received different COVID-19 vaccine schedules. The study compared subjects primed with adenoviral ChAdOx1-S (ChAd) and boosted with BNT162b2 (BNT) mRNA vaccines (ChAd/BNT) to homologous dosing with BNT/BNT or ChAd/ChAd vaccines. Serum samples were collected at two, four and six months after vaccination, and anti-Spike IgG responses were determined. The heterologous vaccination induced a more robust immune response than the two homologous vaccinations. ChAd/BNT induced a stronger immune response than ChAd/ChAd at all time points, whereas the differences between ChAd/BNT and BNT/BNT decreased over time and were not significant at six months. Furthermore, the kinetic parameters associated with IgG decay were estimated by applying a first-order kinetics equation. ChAd/BNT vaccination was associated with the longest time of anti-S IgG negativization and with a slow decay of the titer over time. Finally, analyzing factors influencing the immune response by ANCOVA analysis, it was found that the vaccine schedule had a significant impact on both the IgG titer and kinetic parameters, and having a Body Mass Index (BMI) above the overweight threshold was associated with an impaired immune response. Overall, the heterologous ChAd/BNT vaccination may offer longer-lasting protection against SARS-CoV-2 than homologous vaccination strategies.
Keywords: COVID-19; SARS-CoV-2; anti-Spike IgG response; antibody decay; heterologous vaccination.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses or interpretation of the data, in the writing of the manuscript or in the decision to publish the results.
Figures

References
-
- Barocci S., Orlandi C., Diotallevi A., Buffi G., Ceccarelli M., Vandini D., Carlotti E., Galluzzi L., Rocchi M.B.L., Magnani M., et al. Evaluation of Two-Month Antibody Levels after Heterologous ChAdOx1-S/BNT162b2 Vaccination Compared to Homologous ChAdOx1-S or BNT162b2 Vaccination. Vaccines. 2022;10:491. doi: 10.3390/vaccines10040491. - DOI - PMC - PubMed
-
- LIAISON® SARS-CoV-2 TrimericS IgG Assay. [(accessed on 21 March 2023)]. Available online: https://www.diasorin.com/sites/default/files/allegati_prodotti/liaisonr_....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

