Apical-Out Human Airway Organoids Modeling SARS-CoV-2 Infection
- PMID: 37243252
- PMCID: PMC10220522
- DOI: 10.3390/v15051166
Apical-Out Human Airway Organoids Modeling SARS-CoV-2 Infection
Abstract
The respiratory epithelium, particularly the airway epithelium, is the primary infection site for respiratory pathogens. The apical surface of epithelial cells is constantly exposed to external stimuli including invading pathogens. Efforts have been made to establish organoid cultures to recapitulate the human respiratory tract. However, a robust and simple model with an easily accessible apical surface would benefit respiratory research. Here, we report the generation and characterization of apical-out airway organoids from the long-term expandable lung organoids that we previously established. The apical-out airway organoids morphologically and functionally recapitulated the human airway epithelium at a comparable level to the apical-in airway organoids. Moreover, apical-out airway organoids sustained productive and multicycle replication of SARS-CoV-2, and accurately recapitulated the higher infectivity and replicative fitness of the Omicron variants BA.5 and B.1.1.529 and an ancestral virus. In conclusion, we established a physiologically relevant and convenient apical-out airway organoid model for studying respiratory biology and diseases.
Keywords: Omicron variant; SARS-CoV-2; epithelial polarity; human airway organoids.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

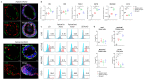
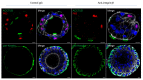
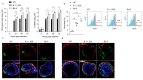
Similar articles
-
Human Nasal Organoids Model SARS-CoV-2 Upper Respiratory Infection and Recapitulate the Differential Infectivity of Emerging Variants.mBio. 2022 Aug 30;13(4):e0194422. doi: 10.1128/mbio.01944-22. Epub 2022 Aug 8. mBio. 2022. PMID: 35938726 Free PMC article.
-
A bipotential organoid model of respiratory epithelium recapitulates high infectivity of SARS-CoV-2 Omicron variant.Cell Discov. 2022 Jun 17;8(1):57. doi: 10.1038/s41421-022-00422-1. Cell Discov. 2022. PMID: 35710786 Free PMC article.
-
The Human Nose Organoid Respiratory Virus Model: an Ex Vivo Human Challenge Model To Study Respiratory Syncytial Virus (RSV) and Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Pathogenesis and Evaluate Therapeutics.mBio. 2021 Feb 22;13(1):e0351121. doi: 10.1128/mbio.03511-21. Epub 2022 Feb 15. mBio. 2021. PMID: 35164569 Free PMC article.
-
[Development of human iPS cell-derived alveolar and airway models using micropatterning plate for SARS-CoV-2 research].Uirusu. 2024;74(1):35-44. doi: 10.2222/jsv.74.35. Uirusu. 2024. PMID: 39617451 Review. Japanese.
-
Airway and Alveoli Organoids as Valuable Research Tools in COVID-19.ACS Biomater Sci Eng. 2021 Aug 9;7(8):3487-3502. doi: 10.1021/acsbiomaterials.1c00306. Epub 2021 Jul 21. ACS Biomater Sci Eng. 2021. PMID: 34288642 Review.
Cited by
-
Advanced lung organoids for respiratory system and pulmonary disease modeling.J Tissue Eng. 2024 Feb 22;15:20417314241232502. doi: 10.1177/20417314241232502. eCollection 2024 Jan-Dec. J Tissue Eng. 2024. PMID: 38406820 Free PMC article. Review.
-
Antimalarial compounds exhibit variant- and cell-type-specific activity against SARS-CoV-2 isolated in Panama.Front Pharmacol. 2025 Jun 4;16:1537053. doi: 10.3389/fphar.2025.1537053. eCollection 2025. Front Pharmacol. 2025. PMID: 40535766 Free PMC article.
-
SARS-CoV-2 Omicron entry is type II transmembrane serine protease-mediated in human airway and intestinal organoid models.J Virol. 2023 Aug 31;97(8):e0085123. doi: 10.1128/jvi.00851-23. Epub 2023 Aug 9. J Virol. 2023. PMID: 37555660 Free PMC article.
-
Human respiratory organoids sustained reproducible propagation of human rhinovirus C and elucidation of virus-host interaction.Nat Commun. 2024 Dec 30;15(1):10772. doi: 10.1038/s41467-024-55076-2. Nat Commun. 2024. PMID: 39738014 Free PMC article.
-
Human Lung Organoids-A Novel Experimental and Precision Medicine Approach.Cells. 2023 Aug 15;12(16):2067. doi: 10.3390/cells12162067. Cells. 2023. PMID: 37626876 Free PMC article. Review.
References
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

