In Silico and In Vitro Evaluation of Some Amidine Derivatives as Hit Compounds towards Development of Inhibitors against Coronavirus Diseases
- PMID: 37243257
- PMCID: PMC10223987
- DOI: 10.3390/v15051171
In Silico and In Vitro Evaluation of Some Amidine Derivatives as Hit Compounds towards Development of Inhibitors against Coronavirus Diseases
Abstract
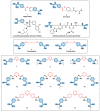
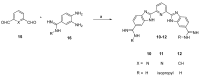
Coronaviruses, including SARS-CoV-2, SARS-CoV, MERS-CoV and influenza A virus, require the host proteases to mediate viral entry into cells. Rather than targeting the continuously mutating viral proteins, targeting the conserved host-based entry mechanism could offer advantages. Nafamostat and camostat were discovered as covalent inhibitors of TMPRSS2 protease involved in viral entry. To circumvent their limitations, a reversible inhibitor might be required. Considering nafamostat structure and using pentamidine as a starting point, a small set of structurally diverse rigid analogues were designed and evaluated in silico to guide selection of compounds to be prepared for biological evaluation. Based on the results of in silico study, six compounds were prepared and evaluated in vitro. At the enzyme level, compounds 10-12 triggered potential TMPRSS2 inhibition with low micromolar IC50 concentrations, but they were less effective in cellular assays. Meanwhile, compound 14 did not trigger potential TMPRSS2 inhibition at the enzyme level, but it showed potential cellular activity regarding inhibition of membrane fusion with a low micromolar IC50 value of 10.87 µM, suggesting its action could be mediated by another molecular target. Furthermore, in vitro evaluation showed that compound 14 inhibited pseudovirus entry as well as thrombin and factor Xa. Together, this study presents compound 14 as a hit compound that might serve as a starting point for developing potential viral entry inhibitors with possible application against coronaviruses.
Keywords: antiviral agents; coronaviruses; viral entry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The TMPRSS2 Inhibitor Nafamostat Reduces SARS-CoV-2 Pulmonary Infection in Mouse Models of COVID-19.mBio. 2021 Aug 31;12(4):e0097021. doi: 10.1128/mBio.00970-21. Epub 2021 Aug 3. mBio. 2021. PMID: 34340553 Free PMC article.
-
Synergistic Block of SARS-CoV-2 Infection by Combined Drug Inhibition of the Host Entry Factors PIKfyve Kinase and TMPRSS2 Protease.J Virol. 2021 Oct 13;95(21):e0097521. doi: 10.1128/JVI.00975-21. Epub 2021 Aug 18. J Virol. 2021. PMID: 34406858 Free PMC article.
-
In Silico Analysis and Synthesis of Nafamostat Derivatives and Evaluation of Their Anti-SARS-CoV-2 Activity.Viruses. 2022 Feb 14;14(2):389. doi: 10.3390/v14020389. Viruses. 2022. PMID: 35215982 Free PMC article.
-
Current status of antivirals and druggable targets of SARS CoV-2 and other human pathogenic coronaviruses.Drug Resist Updat. 2020 Dec;53:100721. doi: 10.1016/j.drup.2020.100721. Epub 2020 Aug 26. Drug Resist Updat. 2020. PMID: 33132205 Free PMC article. Review.
-
The discovery and development of transmembrane serine protease 2 (TMPRSS2) inhibitors as candidate drugs for the treatment of COVID-19.Expert Opin Drug Discov. 2022 Mar;17(3):231-246. doi: 10.1080/17460441.2022.2029843. Epub 2022 Jan 24. Expert Opin Drug Discov. 2022. PMID: 35072549 Free PMC article. Review.
Cited by
-
Design, Synthesis, In Vitro, and In Silico Studies of New N5-Substituted-pyrazolo[3,4-d]pyrimidinone Derivatives as Anticancer CDK2 Inhibitors.Pharmaceuticals (Basel). 2023 Nov 11;16(11):1593. doi: 10.3390/ph16111593. Pharmaceuticals (Basel). 2023. PMID: 38004458 Free PMC article.
-
Small Molecules Incorporating Privileged Amidine Moiety as Potential Hits Combating Antibiotic-Resistant Bacteria.Pharmaceuticals (Basel). 2023 Jul 22;16(7):1040. doi: 10.3390/ph16071040. Pharmaceuticals (Basel). 2023. PMID: 37513951 Free PMC article.
-
Novel Antiviral Agents: Synthesis, Molecular Modelling Studies and Biological Investigation.Viruses. 2023 Oct 2;15(10):2042. doi: 10.3390/v15102042. Viruses. 2023. PMID: 37896819 Free PMC article.
-
Amidine containing compounds: Antimicrobial activity and its potential in combating antimicrobial resistance.Heliyon. 2024 May 31;10(15):e32010. doi: 10.1016/j.heliyon.2024.e32010. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39170404 Free PMC article. Review.
References
-
- Notarte K.I., Catahay J.A., Velasco J.V., Pastrana A., Ver A.T., Pangilinan F.C., Peligro P.J., Casimiro M., Guerrero J.J., Gellaco M.M.L., et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: A systematic review. EClinicalMedicine. 2022;53:101624. doi: 10.1016/j.eclinm.2022.101624. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

