Regional Glymphatic Abnormality in Behavioral Variant Frontotemporal Dementia
- PMID: 37243334
- PMCID: PMC10657235
- DOI: 10.1002/ana.26710
Regional Glymphatic Abnormality in Behavioral Variant Frontotemporal Dementia
Erratum in
-
Correction: Regional Glymphatic Abnormality in Behavioral Variant Frontotemporal Dementia.Ann Neurol. 2023 Oct 20. doi: 10.1002/ana.26806. Online ahead of print. Ann Neurol. 2023. PMID: 37860967 No abstract available.
Abstract
Objectives: Glymphatic function has not yet been explored in behavioral variant frontotemporal dementia (bvFTD). The spatial correlation between regional glymphatic function and bvFTD remains unknown.
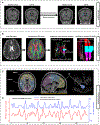
Method: A total of 74 patients with bvFTD and 67 age- and sex-matched healthy controls (HCs) were selected from discovery dataset and replication dataset. All participants underwent neuropsychological assessment. Glymphatic measures including choroid plexus (CP) volume, diffusion tensor imaging along the perivascular (DTI-ALPS) index, and coupling between blood-oxygen-level-dependent signals and cerebrospinal fluid signals (BOLD-CSF coupling), were compared between the two groups. Regional glymphatic function was evaluated by dividing DTI-ALPS and BOLD-CSF coupling into anterior, middle, and posterior regions. The bvFTD-related metabolic pattern was identified using spatial covariance analysis based on l8 F-FDG-PET.
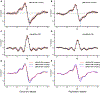
Results: Patients with bvFTD showed higher CP volume (p < 0.001); anterior and middle DTI-ALPS (p < 0.001); and weaker anterior BOLD-CSF coupling (p < 0.05) than HCs after controlling for cortical gray matter volume in both datasets. In bvFTD from the discovery dataset, the anterior DTI-ALPS was negatively associated with the expression of the bvFTD-related metabolic pattern (r = -0.52, p = 0.034) and positively related with regional standardized uptake value ratios of l8 F-FDG-PET in bvFTD-related brain regions (r range: 0.49 to 0.62, p range: 0.017 to 0.047). Anterior and middle glymphatic functions were related to global cognition and disease severity.
Interpretation: Our findings reveal abnormal glymphatic function, especially in the anterior and middle regions of brain in bvFTD. Regional glymphatic dysfunction may contribute to the pathogenesis of bvFTD. ANN NEUROL 2023;94:442-456.
© 2023 The Authors. Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Potential Conflicts of Interest
The authors have no conflicts of interest to declare.
Figures
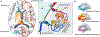



Comment in
-
Glymphatic dysfunction detected in bvFTD.Nat Rev Neurol. 2023 Jul;19(7):387. doi: 10.1038/s41582-023-00834-x. Nat Rev Neurol. 2023. PMID: 37280290 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

