Randomized Placebo-Controlled Trial to Evaluate Effects of Eplerenone on Myocardial Perfusion and Function Among Persons With Human Immunodeficiency Virus (HIV)-Results From the MIRACLE HIV Study
- PMID: 37243345
- PMCID: PMC10573745
- DOI: 10.1093/cid/ciad310
Randomized Placebo-Controlled Trial to Evaluate Effects of Eplerenone on Myocardial Perfusion and Function Among Persons With Human Immunodeficiency Virus (HIV)-Results From the MIRACLE HIV Study
Abstract
Background: Increased renin angiotensin aldosterone system (RAAS) activity may contribute to excess cardiovascular disease in people with HIV (PWH). We investigated how RAAS blockade may improve myocardial perfusion, injury, and function among well-treated PWH.
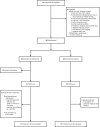
Methods: Forty PWH, on stable ART, without known heart disease were randomized to eplerenone 50 mg PO BID (n = 20) or identical placebo (n = 20) for 12 months. The primary endpoints were (1) myocardial perfusion assessed by coronary flow reserve (CFR) on cardiac PET or stress myocardial blood flow (sMBF) on cardiac MRI or (2) myocardial inflammation by extracellular mass index (ECMi) on cardiac MRI.
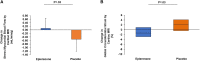
Results: Beneficial effects on myocardial perfusion were seen for sMBF by cardiac MRI (mean [SD]: 0.09 [0.56] vs -0.53 [0.68] mL/min/g; P = .03) but not CFR by cardiac PET (0.01 [0.64] vs -0.07 [0.48]; P = .72, eplerenone vs placebo). Eplerenone improved parameters of myocardial function on cardiac MRI including left ventricular end diastolic volume (-13 [28] vs 10 [26] mL; P = .03) and global circumferential strain (GCS; median [interquartile range 25th-75th]: -1.3% [-2.9%-1.0%] vs 2.3% [-0.4%-4.1%]; P = .03), eplerenone versus placebo respectively. On cardiac MRI, improvement in sMBF related to improvement in global circumferential strain (ρ = -0.65, P = .057) among those treated with eplerenone. Selecting for those with impaired myocardial perfusion (CFR <2.5 and/or sMBF <1.8), there was a treatment effect of eplerenone versus placebo to improve CFR (0.28 [0.27] vs -0.05 [0.36]; P = .04). Eplerenone prevented a small increase in troponin (0.00 [-0.13-0.00] vs 0.00 [0.00-0.74] ng/L; P = .03) without effects on ECMi (0.9 [-2.3-4.3] vs -0.7 [-2.2--0.1] g/m2; P = .38). CD4+ T-cell count (127 [-38-286] vs -6 [-168-53] cells/μL; P = .02) increased in the eplerenone- versus placebo-treated groups.
Conclusions: RAAS blockade with eplerenone benefitted key indices and prevented worsening of myocardial perfusion, injury, and function among PWH with subclinical cardiac disease when compared with placebo.
Clinical trials registration: NCT02740179 (https://clinicaltrials.gov/ct2/show/NCT02740179?term=NCT02740179&draw=2&rank=1).
Keywords: HIV; eplerenone; myocardial blood flow; myocardial dysfunction; renin-angiotensin-aldosterone system.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. S. S. was the recipient of a Gilead Sciences Research Scholars award. M. T. L. has received research funding from KOWA, MedImmune, AstraZeneca, Ionis, and Johnson & Johnson Innovation. T. H. B. is a paid member of the Scientific Advisory Board and has equity in Excision BioTherapeutics, Inc. C. R. d. receives consulting fees from Abbott Diagnostics, Quidel/Ortho, Roche Diagnostics, and Siemens Diagnostics. G. K. R. has received trials support from Leonard Meron Biosciences and consulting fees from Teradyne for clinical trial design and from SEED for COVID consulting. He is also an unpaid panel member of the Department of Health and Human Services (DHHS) Opportunistic Infections guidelines review committee and has provided expert medical review testimony for Tufts Medical Center. G. K. R. also reports unpaid participation on a Data Safety Monitoring Board or Advisory Board for Arterial Inflammation and Coronary Microvascular Dysfunction among Women with HIV: Missing Pieces to the MI Risk Puzzle (grant number R01HL146267). S. K. G. has received research funding from KOWA, Gilead, ViiV, and Theratechnologies and received consulting fees from Theratechnologies and ViiV. He is a member of the Scientific Advisory Board of Marathon Asset Management. M. D. reports grants or contracts paid to institutions from Gilead Sciences and Spectrum Dynamics; consulting fees from Sanofi and MedTrace Pharma; and receipt of equipment, materials, drugs, medical writing, gifts, or other services from Amgen. All disclosures are unrelated to this manuscript. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

