Bacterial Oncotraits Rather than Spatial Organization Are Associated with Dysplasia in Ulcerative Colitis
- PMID: 37243505
- PMCID: PMC10673813
- DOI: 10.1093/ecco-jcc/jjad092
Bacterial Oncotraits Rather than Spatial Organization Are Associated with Dysplasia in Ulcerative Colitis
Abstract
Background and aims: Colonic bacterial biofilms are frequently present in ulcerative colitis [UC] and may increase dysplasia risk through pathogens expressing oncotraits. This prospective cohort study aimed to determine [1] the association of oncotraits and longitudinal biofilm presence with dysplasia risk in UC, and [2] the relation of bacterial composition with biofilms and dysplasia risk.
Methods: Faeces and left- and right-sided colonic biopsies were collected from 80 UC patients and 35 controls. Oncotraits [FadA of Fusobacterium, BFT of Bacteroides fragilis, colibactin [ClbB] and Intimin [Eae] of Escherichia coli] were assessed in faecal DNA with multiplex quantitative polymerase chain reaction [qPCR]. Biopsies were screened for biofilms [n = 873] with 16S rRNA fluorescent in situ hybridiation. Shotgun metagenomic sequencing [n = 265], and ki67-immunohistochemistry were performed. Associations were determined with a mixed-effects regression model.
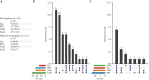
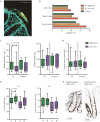
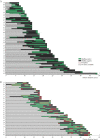
Results: Biofilms were highly prevalent in UC patients [90.8%] with a median persistence of 3 years (interquartile range [IQR] 2-5 years). Biofilm-positive biopsies showed increased epithelial hypertrophy [p = 0.025] and a reduced Shannon diversity independent of disease status [p = 0.015], but were not significantly associated with dysplasia in UC: adjusted odds ratio [aOR] 1.45, 95% confidence interval [CI] 0.63-3.40. In contrast, ClbB independently associated with dysplasia [aOR 7.16, 95% CI 1.75-29.28], and FadA and Fusobacteriales were associated with a decreased dysplasia risk in UC [aOR 0.23, 95% CI 0.06-0.83, p <0.01].
Conclusions: Biofilms are a hallmark of UC; however, because of their high prevalence are a poor biomarker for dysplasia. In contrast, colibactin presence and FadA absence independently associate with dysplasia in UC and might therefore be valuable biomarkers for future risk stratification and intervention strategies.
Keywords: Microbiology; biomarkers; pathology.
© The Author(s) 2023. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Jess T, Rungoe C, Peyrin-Biroulet L.. Risk of colorectal cancer in patients with ulcerative colitis: A meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol 2012;10:639–45. - PubMed
-
- Lutgens MW, van Oijen MG, van der Heijden GJ, Vleggaar FP, Siersema PD, Oldenburg B.. Declining risk of colorectal cancer in inflammatory bowel disease: An updated meta-analysis of population-based cohort studies. Inflamm Bowel Dis 2013;19:789–99. - PubMed
-
- Wintjens DSJ, Bogie RMM, van den Heuvel TRA, et al. Incidence and classification of postcolonoscopy colorectal cancers in inflammatory bowel disease: A Dutch population-based cohort study. J Crohns Colitis 2018;12:777–83. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

