Molecular recognition requires dimerization of a VHH antibody
- PMID: 37243579
- PMCID: PMC10228392
- DOI: 10.1080/19420862.2023.2215363
Molecular recognition requires dimerization of a VHH antibody
Abstract
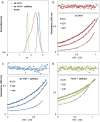
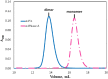
Camelid heavy-chain-only antibodies are a unique class of antibody that possesses only a single variable domain (termed VHH) for antigen recognition. Despite their apparent canonical mechanism of target recognition, where a single VHH domain binds a single target, an anti-caffeine VHH has been observed to possess 2:1 stoichiometry. Here, the structure of the anti-caffeine VHH/caffeine complex enabled the generation and biophysical analysis of variants that were used to better understand the role of VHH homodimerization in caffeine recognition. VHH interface mutants and caffeine analogs, which were examined to probe the mechanism of caffeine binding, suggested caffeine recognition is only possible with the VHH dimer species. Correspondingly, in the absence of caffeine, the anti-caffeine VHH was found to form a dimer with a dimerization constant comparable to that observed with VH:VL domains in conventional antibody systems, which was most stable near physiological temperature. While the VHH:VHH dimer structure (at 1.13 Å resolution) is reminiscent of conventional VH:VL heterodimers, the homodimeric VHH possesses a smaller angle of domain interaction, as well as a larger amount of apolar surface area burial. To test the general hypothesis that the short complementarity-determining region-3 (CDR3) may help drive VHH:VHH homodimerization, an anti-picloram VHH domain containing a short CDR3 was generated and characterized, which revealed it also existed as dimer species in solution. These results suggest homodimer-driven recognition may represent a more common method of VHH ligand recognition, opening opportunities for novel VHH homodimer affinity reagents and helping to guide their use in chemically induced dimerization applications.
Keywords: Camelid antibody; ITC; VHH; chemically induced dimerization (CID) systems; crystal structure; dimerization; hapten recognition; nanobody; sdAb.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures




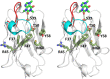
References
-
- Decanniere K, Desmyter A, Lauwereys M, Ghahroudi MA, Muyldermans S, Wyns L. A single-domain antibody fragment in complex with RNase A: non-canonical loop structures and nanomolar affinity using two CDR loops. Structure. 1999;7(4):361–70. PMID: 10196124. doi: 10.1016/s0969-2126(99)80049-5. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
