Role of mitochondria in the myopathy of juvenile dermatomyositis and implications for skeletal muscle calcinosis
- PMID: 37244073
- PMCID: PMC10330803
- DOI: 10.1016/j.jaut.2023.103061
Role of mitochondria in the myopathy of juvenile dermatomyositis and implications for skeletal muscle calcinosis
Abstract
Objectives: To elucidate mechanisms contributing to skeletal muscle calcinosis in patients with juvenile dermatomyositis.
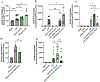
Methods: A well-characterized cohorts of JDM (n = 68), disease controls (polymyositis, n = 7; juvenile SLE, n = 10, and RNP + overlap syndrome, n = 12), and age-matched health controls (n = 17) were analyzed for circulating levels of mitochondrial (mt) markers including mtDNA, mt-nd6, and anti-mitochondrial antibodies (AMAs) using standard qPCR, ELISA, and novel-in-house assays, respectively. Mitochondrial calcification of affected tissue biopsies was confirmed using electron microscopy and energy dispersive X-ray analysis. A human skeletal muscle cell line, RH30, was used to generate an in vitro calcification model. Intracellular calcification is measured by flow cytometry and microscopy. Mitochondria were assessed for mtROS production and membrane potential by flow cytometry and real-time oxygen consumption rate by Seahorse bioanalyzer. Inflammation (interferon-stimulated genes) was measured by qPCR.
Results: In the current study, patients with JDM exhibited elevated levels of mitochondrial markers associated with muscle damage and calcinosis. Of particular interest are AMAs predictive of calcinosis. Human skeletal muscle cells undergo time- and dose-dependent accumulation of calcium phosphate salts with preferential localization to mitochondria. Calcification renders skeletal muscle cells mitochondria stressed, dysfunctional, destabilized, and interferogenic. Further, we report that inflammation induced by interferon-alpha amplifies mitochondrial calcification of human skeletal muscle cells via the generation of mitochondrial reactive oxygen species (mtROS).
Conclusions: Overall, our study demonstrates the mitochondrial involvement in the skeletal muscle pathology and calcinosis of JDM and mtROS as a central player in the calcification of human skeletal muscle cells. Therapeutic targeting of mtROS and/or upstream inducers, such as inflammation, may alleviate mitochondrial dysfunction, leading to calcinosis. AMAs can potentially identify patients with JDM at risk for developing calcinosis.
Keywords: Calcinosis; Interferon; Juvenile dermatomyositis; Mitochondria; Mitochondrial reactive oxygen species.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest None declared.
Figures
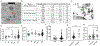

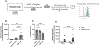


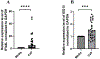
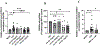
References
-
- Symmons DP, Sills JA, Davis SM, The incidence of juvenile dermatomyositis: results from a nation-wide study, Br. J. Rheumatol. 34 (1995) 732–736. - PubMed
-
- Feldman BM, Rider LG, Reed AM, Pachman LM, Juvenile dermatomyositis and other idiopathic inflammatory myopathies of childhood, Lancet 371 (2008) 2201–2212. - PubMed
-
- Pachman LM, Boskey AL, Clinical manifestations and pathogenesis of hydroxyapatite crystal deposition in juvenile dermatomyositis, Curr. Rheumatol. Rep. 8 (2006) 236–243. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

