Optimal dosing of heparin for prophylactic anticoagulation in critically ill COVID-19 patients a systematic review and meta-analysis of randomized controlled trials
- PMID: 37244209
- PMCID: PMC10211463
- DOI: 10.1016/j.jcrc.2023.154344
Optimal dosing of heparin for prophylactic anticoagulation in critically ill COVID-19 patients a systematic review and meta-analysis of randomized controlled trials
Abstract
Purpose: The optimal amount of anticoagulation for critically ill COVID-19 patients is controversial. Therefore, we aimed to evaluate the efficacy and safety of escalated doses of anticoagulation in critically ill patients with severe COVID-19.
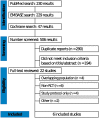
Materials and methods: We conducted a systematic search of three major databases, including PubMed, Cochrane Library, and Embase, from inception to May 2022. Randomized controlled trials (RCTs) were included comparing therapeutic or intermediate doses to standard prophylactic doses of anticoagulants in critically ill COVID-19 patients, with heparins as the only anticoagulation therapy considered.
Results: Out of the six RCTs, 2130 patients were administered escalated dose anticoagulation (50.2%) and standard thromboprophylaxis therapy (49.8%). The escalated dose showed no significant impact on mortality (RR, 1.01; 95% CI, 0.90-1.13). Although there was no significant difference in DVT (RR, 0.81; 95% CI, 0.61-1.08), the risk of PE was significantly reduced in patients receiving escalated dose anticoagulation (RR, 0.35; 95% CI, 0.21-0.60), with an increased risk of bleeding events (RR, 1.65; 95% CI, 1.08-2.53).
Conclusion: This systematic review and meta-analysis fail to support escalated anticoagulation doses to reduce mortality in critically ill COVID-19 patients. However, higher doses of anticoagulants appear to reduce thrombotic events while increasing the risk of bleeding effectively.
Keywords: Anticoagulants; Bleeding; COVID-19; Critical care; Heparin; Hypercoagulability; Intensive care unit; Mortality; SARS-CoV2; Safety; Venous thromboembolism; meta-analysis.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest Not declared.
Figures



Comment in
-
The standard of care is standard for a reason: Commentary on "Optimal dosing of heparin for prophylactic anticoagulation in critically ill COVID-19 patients: A systematic review and Meta-analysis of randomized clinical trials".J Crit Care. 2023 Oct;77:154345. doi: 10.1016/j.jcrc.2023.154345. Epub 2023 May 25. J Crit Care. 2023. PMID: 37244208 Free PMC article. No abstract available.
References
-
- COVID-19 Treatment Guidelines Panel . National Institutes of Health; 2022. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines.https://www.covid19treatmentguidelines.nih.gov/ Accessed September 23. Available at. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

