Efficacy and safety of mRNA and AstraZeneca COVID-19 vaccines in patients with autoimmune rheumatic diseases: A systematic review
- PMID: 37244811
- PMCID: PMC10201317
- DOI: 10.1016/j.vaccine.2023.05.048
Efficacy and safety of mRNA and AstraZeneca COVID-19 vaccines in patients with autoimmune rheumatic diseases: A systematic review
Abstract
Background: Patients with autoimmune rheumatic diseases (ARD) are at a potentially higher risk for COVID-19 infection complications. Given their inherent altered immune system and the use of immunomodulatory medications, vaccine immunogenicity could be unpredictable with a suboptimal or even an exaggerated immunological response. The aim of this study is to provide real-time data on the emerging evidence of COVID-19 vaccines' efficacy and safety in patients with ARDs.
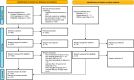
Methods: We performed a literature search of the PubMed, EMBASE, and OVID databases up to 11-13 April 2022 on the efficacy and safety of both types of the mRNA-vaccines and the AstraZeneca COVID-19 vaccines in patients with ARD. The risk of bias in the retrieved studies was evaluated using the Quality in Prognostic Studies tool. Also, current clinical practice guidelines from multiple international professional societies were reviewed.
Results: We identified 60 prognostic studies, 69 case reports and case series, and eight international clinical practice guidelines. Our results demonstrated that most patients with ARDs were able to mount humoral and/or cellular responses after two doses of COVID-19 vaccine although this response was suboptimal in patients receiving certain disease-modifying medications including rituximab, methotrexate, mycophenolate mofetil, daily glucocorticoids >10 mg, abatacept, as well as in older individuals, and those with comorbid interstitial lung diseases. Safety reports on COVID-19 vaccines in patients with ARDs were largely reassuring with mostly self-limiting adverse events and very minimal post-vaccination disease flares.
Conclusion: Both types of the mRNA-vaccines and the AstraZeneca COVID-19 vaccines are highly effective and safe in patients with ARD. However, due to their suboptimal response in some patients, alternative mitigation strategies such as booster vaccines and shielding practices should also be followed. Management of immunomodulatory treatment regimens during the peri vaccination period should be individualized through shared decision making with patients and their attending rheumatologists.
Keywords: Adverse events; Autoimmune Rheumatic Disease; COVID-19 vaccine; Disease flare; Immunogenicity; Safety.
Copyright © 2023 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Efficacy and safety of COVID-19 vaccines.Cochrane Database Syst Rev. 2022 Dec 7;12(12):CD015477. doi: 10.1002/14651858.CD015477. Cochrane Database Syst Rev. 2022. PMID: 36473651 Free PMC article.
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Safety, efficacy, and immunogenicity of SARS-CoV-2 mRNA vaccination in children and adult patients with rheumatic diseases: a comprehensive literature review.Rheumatol Int. 2024 Dec;44(12):2757-2794. doi: 10.1007/s00296-024-05734-x. Epub 2024 Nov 22. Rheumatol Int. 2024. PMID: 39576327
Cited by
-
Adenovirus Transcriptome in Human Cells Infected with ChAdOx1-Vectored Candidate HIV-1 Vaccine Is Dominated by High Levels of Correctly Spliced HIVconsv1&62 Transgene RNA.Vaccines (Basel). 2023 Jul 1;11(7):1187. doi: 10.3390/vaccines11071187. Vaccines (Basel). 2023. PMID: 37515003 Free PMC article.
-
Efficacy, Immunogenicity, and Safety of COVID-19 Vaccines in Patients with Autoimmune Diseases: A Systematic Review and Meta-Analysis.Vaccines (Basel). 2023 Sep 4;11(9):1456. doi: 10.3390/vaccines11091456. Vaccines (Basel). 2023. PMID: 37766132 Free PMC article. Review.
-
COVID-19 Vaccination Recommendations for Immunocompromised Patient Populations: Delphi Panel and Consensus Statement Generation in the United States.Infect Dis Ther. 2024 Nov;13(11):2255-2283. doi: 10.1007/s40121-024-01052-8. Epub 2024 Oct 10. Infect Dis Ther. 2024. PMID: 39387989 Free PMC article.
-
Association of COVID-19 Vaccinations With Flares of Systemic Rheumatic Disease: A Case-Crossover Study.Arthritis Care Res (Hoboken). 2024 May;76(5):733-742. doi: 10.1002/acr.25288. Epub 2024 Feb 7. Arthritis Care Res (Hoboken). 2024. PMID: 38163750 Free PMC article.
-
Flares of Systemic Autoimmune Rheumatic Disease Following Coronavirus Disease 2019 Vaccination: A Narrative Review.Rheum Dis Clin North Am. 2025 Feb;51(1):75-92. doi: 10.1016/j.rdc.2024.08.005. Epub 2024 Oct 15. Rheum Dis Clin North Am. 2025. PMID: 39550108 Review.
References
-
- Middleton's Allergy: Principles and Practice. 9th ed. Elsevier; 2022. ClinicalKey. Available at: https://www.clinicalkey.com/.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

