Hypovolemia with peripheral edema: What is wrong?
- PMID: 37245039
- PMCID: PMC10225095
- DOI: 10.1186/s13054-023-04496-5
Hypovolemia with peripheral edema: What is wrong?
Abstract
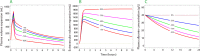
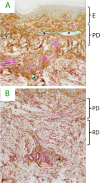
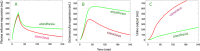
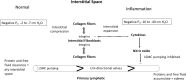
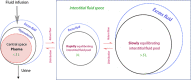
Fluid normally exchanges freely between the plasma and interstitial space and is returned primarily via the lymphatic system. This balance can be disturbed by diseases and medications. In inflammatory disease states, such as sepsis, the return flow of fluid from the interstitial space to the plasma seems to be very slow, which promotes the well-known triad of hypovolemia, hypoalbuminemia, and peripheral edema. Similarly, general anesthesia, for example, even without mechanical ventilation, increases accumulation of infused crystalloid fluid in a slowly equilibrating fraction of the extravascular compartment. Herein, we have combined data from fluid kinetic trials with previously unconnected mechanisms of inflammation, interstitial fluid physiology and lymphatic pathology to synthesize a novel explanation for common and clinically relevant examples of circulatory dysregulation. Experimental studies suggest that two key mechanisms contribute to the combination of hypovolemia, hypoalbuminemia and edema; (1) acute lowering of the interstitial pressure by inflammatory mediators such as TNFα, IL-1β, and IL-6 and, (2) nitric oxide-induced inhibition of intrinsic lymphatic pumping.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Guyton AC, Hall JE. The body fluid compartments: extracellular and intracellular fluids; interstitial fluid and edema. In: Textbook of medical physiology, 9th edn. Philadelphia: W.B. Saunders Company; 1996. p. 311–312.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

