A Polygenic Risk Score for Predicting Racial and Genetic Susceptibility to Prurigo Nodularis
- PMID: 37245863
- PMCID: PMC11290854
- DOI: 10.1016/j.jid.2023.04.033
A Polygenic Risk Score for Predicting Racial and Genetic Susceptibility to Prurigo Nodularis
Abstract
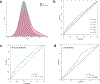
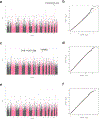
Prurigo nodularis (PN) is an understudied inflammatory skin disease characterized by pruritic, hyperkeratotic nodules. Identifying the genetic factors underlying PN could help to better understand its etiology and guide the development of therapies. In this study, we developed a polygenic risk score that predicts a diagnosis of PN (OR = 1.41, P = 1.6 × 10-5) in two independent and continentally distinct populations. We also performed GWASs, which uncovered genetic variants associated with PN, including one near PLCB4 (rs6039266: OR = 3.15, P = 4.8 × 10-8) and others near TXNRD1 (rs34217906: OR = 1.71, P = 6.4 × 10-7; rs7134193: OR = 1.57, P = 1.1 × 10-6). Finally, we discovered that Black patients have over a two-times greater genetic risk of developing PN (OR = 2.63, P = 7.8 × 10-4). Combining the polygenic risk score and self-reported race together was significantly predictive of PN (OR = 1.32, P = 4.7 × 10-3). Strikingly, this association was more significant with race than after adjusting for genetic ancestry. Because race is a sociocultural construct and not a genetically bound category, our findings suggest that genetics, environmental influence, and social determinants of health likely affect the development of PN and may contribute to clinically observed racial disparities.
Copyright © 2023. Published by Elsevier Inc.
Conflict of interest statement
Conflicts of Interest and Financial Disclosures
Dr. Vasavda is a paid consultant and stockholder of AstraZeneca. Dr. Kwatra is an advisory board member/consultant for Abbvie, Aslan Pharmaceuticals, Arcutis Biotherapeutics, Castle Biosciences, Celldex Therapeutics, Galderma, Genzada Pharmaceuticals, Incyte Corporation, Johnson & Johnson, Kiniksa Pharmaceuticals, Leo Pharma, Novartis Pharmaceuticals Corporation, Pfizer, Regeneron Pharmaceuticals, and Sanofi and has served as an investigator for Galderma, Incyte, Pfizer, and Sanofi. The remaining authors state no conflict of interest.
Figures

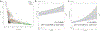
References
-
- Aggarwal P, Choi J, Sutaria N, Roh YS, Wongvibulsin S, Williams KA, et al. Clinical characteristics and disease burden in prurigo nodularis. Clin Exp Dermatol. 2021;46(7):1277–84 - PubMed
-
- Brenaut E, Halvorsen JA, Dalgard FJ, Lien L, Balieva F, Sampogna F, et al. The self-assessed psychological comorbidities of prurigo in European patients: a multicentre study in 13 countries. J Eur Acad Dermatol. 2019;33(1):157–62 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

