Combined spectroscopic and structural approaches to explore the mechanism of histidine-ligated heme-dependent aromatic oxygenases
- PMID: 37245909
- PMCID: PMC11057917
- DOI: 10.1016/bs.mie.2023.03.008
Combined spectroscopic and structural approaches to explore the mechanism of histidine-ligated heme-dependent aromatic oxygenases
Abstract
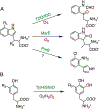
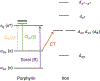
The emergence of histidine-ligated heme-dependent aromatic oxygenases (HDAOs) has greatly enriched heme chemistry, and more studies are required to appreciate the diversity found in His-ligated heme proteins. This chapter describes recent methods in probing the HDAO mechanisms in detail, along with the discussion on how they can benefit structure-function studies of other heme systems. The experimental details are centered on studies of TyrHs, followed by explanation of how the results obtained would advance the understanding of the specific enzyme and also HDAOs. Spectroscopic methods, namely, electronic absorption and EPR spectroscopies, and X-ray crystallography are valuable techniques commonly used to characterize the properties of the heme center and the nature of heme-based intermediate. Herein, we show that the combination of these tools are extremely powerful, not only because one can acquire electronic, magnetic, and conformational information from different phases, but also because of the advantages brought by spectroscopic characterization on crystal samples.
Keywords: Heme-based intermediate; Histidine-ligated heme enzymes; Protein crystallization; Spectroscopy; Substrate analog.
Copyright © 2023 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
A new regime of heme-dependent aromatic oxygenase superfamily.Proc Natl Acad Sci U S A. 2021 Oct 26;118(43):e2106561118. doi: 10.1073/pnas.2106561118. Proc Natl Acad Sci U S A. 2021. PMID: 34667125 Free PMC article.
-
Characterization of heme ligation properties of Rv0203, a secreted heme binding protein involved in Mycobacterium tuberculosis heme uptake.Biochemistry. 2012 Feb 21;51(7):1518-31. doi: 10.1021/bi2018305. Epub 2012 Feb 8. Biochemistry. 2012. PMID: 22283334 Free PMC article.
-
Resonance Raman and EPR spectroscopic studies on heme-heme oxygenase complexes.Biochemistry. 1993 Dec 28;32(51):14151-7. doi: 10.1021/bi00214a012. Biochemistry. 1993. PMID: 8260499
-
The heme environment of mouse neuroglobin: histidine imidazole plane orientations obtained from solution NMR and EPR spectroscopy as compared with X-ray crystallography.J Biol Inorg Chem. 2006 Jun;11(4):391-7. doi: 10.1007/s00775-006-0095-8. Epub 2006 Apr 4. J Biol Inorg Chem. 2006. PMID: 16586113 Review.
-
The power of using continuous-wave and pulsed electron paramagnetic resonance methods for the structure analysis of ferric forms and nitric oxide-ligated ferrous forms of globins.Methods Enzymol. 2008;437:287-310. doi: 10.1016/S0076-6879(07)37015-8. Methods Enzymol. 2008. PMID: 18433634 Review.
References
-
- Ball HJ, Jusof FF, Bakmiwewa SM, Hunt NH, & Yuasa HJ (2014). Tryptophan-catabolizing enzymes—Party of Three. Frontiers in Immunology, 5. https://www.frontiersin.org/articles/10.3389/fimmu.2014.00485. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

