Recent advances in the development of therapeutic peptides
- PMID: 37246037
- PMCID: PMC10330351
- DOI: 10.1016/j.tips.2023.04.003
Recent advances in the development of therapeutic peptides
Abstract
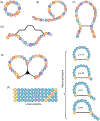
Peptides have unique characteristics that make them highly desirable as therapeutic agents. The physicochemical and proteolytic stability profiles determine the therapeutic potential of peptides. Multiple strategies to enhance the therapeutic profile of peptides have emerged. They include chemical modifications, such as cyclization, substitution with d-amino acids, peptoid formation, N-methylation, and side-chain halogenation, and incorporation in delivery systems. There have been recent advances in approaches to discover peptides having these modifications to attain desirable therapeutic properties. We critically review these recent advancements in therapeutic peptide development.
Keywords: N-methylation; cyclization; d-amino acid; peptides; peptoid; stability.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests The authors have no interests to declare.
Figures


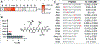
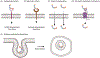
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

