Bicyclic Engineered Sortase A Performs Transpeptidation under Denaturing Conditions
- PMID: 37246906
- PMCID: PMC10288436
- DOI: 10.1021/acs.bioconjchem.3c00151
Bicyclic Engineered Sortase A Performs Transpeptidation under Denaturing Conditions
Abstract
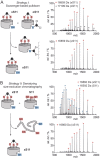
Enzymes are of central importance to many biotechnological and biomedical applications. However, for many potential applications, the required conditions impede enzyme folding and therefore function. The enzyme Sortase A is a transpeptidase that is widely used to perform bioconjugation reactions with peptides and proteins. Thermal and chemical stress impairs Sortase A activity and prevents its application under harsh conditions, thereby limiting the scope for bioconjugation reactions. Here, we report the stabilization of a previously reported, activity-enhanced Sortase A, which suffered from particularly low thermal stability, using the in situ cyclization of proteins (INCYPRO) approach. After introduction of three spatially aligned solvent-exposed cysteines, a triselectrophilic cross-linker was attached. The resulting bicyclic INCYPRO Sortase A demonstrated activity both at elevated temperature and in the presence of chemical denaturants, conditions under which both wild-type Sortase A and the activity-enhanced version are inactive.
Conflict of interest statement
The authors declare the following competing financial interest(s): S.K., G.H.H., A.M., S.H., S.N. and T.N.G. are listed as inventors on patent applications related to the INCYPRO stabilization approach. S.H., S.N. and T.N.G. are co-founders of Incircular B.V. commercializing the INCYPRO technology.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

