mRNA versus inactivated virus COVID-19 vaccines in multiple sclerosis: Humoral responses and protectivity-Does it matter?
- PMID: 37247488
- PMCID: PMC10170898
- DOI: 10.1016/j.msard.2023.104761
mRNA versus inactivated virus COVID-19 vaccines in multiple sclerosis: Humoral responses and protectivity-Does it matter?
Abstract
Background: COVID-19 vaccines are recommended for people with multiple sclerosis (pwMS). Adequate humoral responses are obtained in pwMS receiving disease-modifying therapies (DMTs) after vaccination, with the exception of those receiving B-cell-depleting therapies and non-selective S1P modulators. However, most of the reported studies on the immunity of COVID-19 vaccinations have included mRNA vaccines, and information on inactivated virus vaccine responses, long-term protectivity, and comparative studies with mRNA vaccines are very limited. Here, we aimed to investigate the association between humoral vaccine responses and COVID-19 infection outcomes following mRNA and inactivated virus vaccines in a large national cohort of pwMS receiving DMTs.
Methods: This is a cross-sectional and prospective multicenter study on COVID-19-vaccinated pwMS. Blood samples of pwMS with or without DMTs and healthy controls were collected after two doses of inactivated virus (Sinovac) or mRNA (Pfizer-BioNTech) vaccines. PwMS were sub-grouped according to the mode of action of the DMTs that they were receiving. SARS-CoV-2 IgG titers were evaluated by chemiluminescent microparticle immunoassay. A representative sample of this study cohort was followed up for a year. COVID-19 infection status and clinical outcomes were compared between the mRNA and inactivated virus groups as well as among pwMS subgroups.
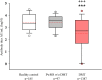
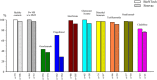
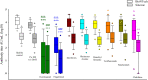
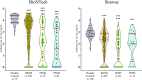
Results: A total of 1484 pwMS (1387 treated, 97 untreated) and 185 healthy controls were included in the analyses (male/female: 544/1125). Of those, 852 (51.05%) received BioNTech, and 817 (48.95%) received Sinovac. mRNA and inactivated virus vaccines result in similar seropositivity; however, the BioNTech vaccination group had significantly higher antibody titers (7.175±10.074) compared with the Sinovac vaccination group (823±1.774) (p<0.001). PwMS under ocrelizumab, fingolimod, and cladribine treatments had lower humoral responses compared with the healthy controls in both vaccine types. After a mean of 327±16 days, 246/704 (34.9%) of pwMS who were contacted had COVID-19 infection, among whom 83% had asymptomatic or mild disease. There was no significant difference in infection rates of COVID-19 between participants vaccinated with BioNTech or Sinovac vaccines. Furthermore, regression analyses show that no association was found regarding age, sex, Expanded Disability Status Scale score (EDSS), the number of vaccination, DMT type, or humoral antibody responses with COVID-19 infection rate and disease severity, except BMI Body mass index (BMI).
Conclusion: mRNA and inactivated virus vaccines had similar seropositivity; however, mRNA vaccines appeared to be more effective in producing SARS-CoV-2 IgG antibodies. B-cell-depleting therapies fingolimod and cladribine were associated with attenuated antibody titer. mRNA and inactive virus vaccines had equal long-term protectivity against COVID-19 infection regardless of the antibody status.
Keywords: COVID-19; Humoral response; Inactivated virus vaccine; Multiple sclerosis; mRNA vaccine.
Copyright © 2023. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest M.Tutuncu, S. Demir, S. Sen, T. Gunduz, C Uzunköprü, H Gumus have received honoraria or consultancy fees for participating to advisory boards, giving educational lectures and/or travel and regis- tration coverage for attending scientific congresses or symposia from F. Hoffmann-La Roche Ltd, Sanofi-Genzyme, Merck-Serono, Novartis, Teva, Biogen Idec/Gen Pharma. Asli Tuncer has received honoraria or consultancy fees for participating to advisory boards, giving educational lectures and/or travel and registration coverage for attending scientific congresses or symposia from F. Hoffmann-La Roche Ltd, Sanofi-Genzyme, Merck-Serono, Novartis, Teva, Biogen Idec/Gen Pharma. Serkan Ozakbas has received honoraria or consultancy fees for participating to advisory boards, giving educational lectures and/or travel and registration coverage for attending scientific congresses or symposia from F. Hoffmann-La Roche Ltd, Sanofi-Genzyme, Merck-Serono, Novartis, Teva, Biogen Idec/Gen Pharma. H. Efendi has received honoraria or consultancy fees for participating to advisory boards, giving educational lectures and/or travel and registration coverage for attending scientific congresses or symposia from F. Hoffmann-La Roche Ltd, Sanofi-Genzyme, Merck-Serono, Novartis, Teva, Biogen Idec/Gen Pharma of Turkey and Abdi Ibrahim Rana Karabudak has received honoraria for giving educational lectures, consultancy fees for participating advisory boards, and travel grants for attending scientific congresses or symposia from Roche, Sanofi-Genzyme, Merck-Serono, Novartis, Teva, Biogen Idec/Gen Pharma of Turkey, Abdi Ibrahim Ilac, Deva and ARIS. Aksel Siva has received honoraria or consultancy fees for participating to advisory boards, giving educational lectures and/or travel and registration coverage for attending scientific congresses or symposia from F. Hoffmann-La Roche Ltd, Sanofi-Genzyme, Merck-Serono, Novartis, Teva, Biogen Idec/Gen Pharma of Turkey and Abdi Ibrahim Ilac. The rest of authors declare no conflict of interest with the study project.
Figures


References
-
- Abbott SARS-COV-2 IMMUNOASSAYS, https://www.corelaboratory.abbott/int/en/offerings/segments/infectious-d....
-
- Achtnichts L., Ovchinnikov A., Jakopp B., et al. SARS-CoV-2 MRNA vaccination in people with multiple sclerosis treated with fingolimod: protective humoral immune responses may develop after the preferred third shot. Vaccines (Basel) 2022;10(2):341. doi: 10.3390/vaccines10020341. 2022 Feb 21. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

