Peripheral sensory neurons and non-neuronal cells express functional Piezo1 channels
- PMID: 37247618
- PMCID: PMC10240879
- DOI: 10.1177/17448069231174315
Peripheral sensory neurons and non-neuronal cells express functional Piezo1 channels
Abstract
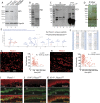
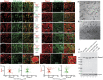
Here, we present evidence showing Piezo1 protein expression in the primary sensory neurons (PSNs) and non-neuronal cells of rat peripheral nervous system. Using a knockdown/knockout validated antibody, we detected Piezo1 immunoreactivity (IR) in ∼60% of PSNs of rat dorsal root ganglia (DRG) with higher IR density in the small- and medium-sized neurons. Piezo1-IR was clearly identified in DRG perineuronal glia, including satellite glial cells (SGCs) and Schwann cells; in sciatic nerve Schwann cells surrounding the axons and cutaneous afferent endings; and in skin epidermal Merkel cells and melanocytes. Neuronal and non-neuronal Piezo1 channels were functional since various cells (dissociated PSNs and SGCs from DRGs, isolated Schwann cells, and primary human melanocytes) exhibited a robust response to Piezo1 agonist Yoda1 by an increase of intracellular Ca2+ concentration ([Ca2+]i). These responses were abolished by non-specific Piezo1 antagonist GsMTx4. Immunoblots showed elevated Piezo1 protein in DRG proximal to peripheral nerve injury-induced painful neuropathy, while PSNs and SGCs from rats with neuropathic pain showed greater Yoda1-evoked elevation of [Ca2+]i and an increased frequency of cells responding to Yoda1, compared to controls. Sciatic nerve application of GsMTx4 alleviated mechanical hypersensitivity induced by Yoda1. Overall, our data show that Piezo1 is widely expressed by the neuronal and non-neuronal cells in the peripheral sensory pathways and that painful nerve injury appeared associated with activation of Piezo1 in PSNs and peripheral glial cells.
Keywords: Piezo-type mechanosensitive ion channel component 1; immunohistochemistry; peripheral nervous system.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures







References
-
- Ranade SS, Qiu Z, Woo SH, Hur SS, Murthy SE, Cahalan SM, Xu J, Mathur J, Bandell M, Coste B, Li YSJ, Chien S, Patapoutian A. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc Natl Acad Sci U S A 2014; 111: 10347–10352. DOI: 10.1073/pnas.1409233111 - DOI - PMC - PubMed
-
- Ranade SS, Woo SH, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Mathur J, Bégay V, Coste B, Mainquist J, Wilson AJ, Francisco AG, Reddy K, Qiu Z, Wood JN, Lewin GR, Patapoutian A. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 2014; 516: 121–125. DOI: 10.1038/nature13980 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

