JAK inhibitors and the risk of malignancy: a meta-analysis across disease indications
- PMID: 37247942
- PMCID: PMC10359573
- DOI: 10.1136/ard-2023-224049
JAK inhibitors and the risk of malignancy: a meta-analysis across disease indications
Abstract
Objectives: To estimate the association of Janus kinase inhibitors (JAKi) with the incidence of malignancy, compared with placebo, tumour necrosis factor (TNF)-α inhibitors (TNFi) and methotrexate.
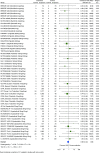
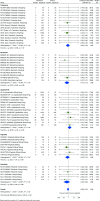
Methods: Systematic searches of databases were performed, to December 2022, to identify phase II/III/IV randomised clinical trials (RCTs) and long-term extension (LTE) studies of JAKi (tofacitinib, baricitinib, upadacitinib, filgotinib, peficitinib) compared with placebo, TNFi or methotrexate, in adults with rheumatoid arthritis, psoriatic arthritis, psoriasis, axial spondyloarthritis, inflammatory bowel disease or atopic dermatitis. Network and pairwise meta-analyses were performed to estimate incidence rate ratios (IRRs) for malignancy between JAKi and comparators. Bias was assessed using the Cochrane Risk of Bias-2 tool.
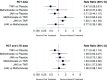
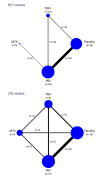
Results: In 62 eligible RCTs and 16 LTE studies, there were 82 366 person-years of exposure to JAKi, 2924 to placebo, 7909 to TNFi and 1074 to methotrexate. The overall malignancy incidence rate was 1.15 per 100 person-years in RCTs, and 1.26 per 100 person-years across combined RCT and LTE data. In network meta-analyses, the incidence of all malignancies including non-melanomatous skin cancers (NMSCs) was not significantly different between JAKi and placebo (IRR 0.71; 95% CI 0.44 to 1.15) or between JAKi and methotrexate (IRR 0.77; 95% CI 0.35 to 1.68). Compared with TNFi, however, JAKi were associated with an increased incidence of malignancy (IRR 1.50; 95% CI 1.16 to 1.94). Findings were consistent when analysing NMSC only and when analysing combined RCT/LTE data.
Conclusions: JAKi were associated with a higher incidence of malignancy compared with TNFi but not placebo or methotrexate. Cancers were rare events in all comparisons.
Prospero registration number: CRD42022362630.
Keywords: arthritis, psoriatic; arthritis, rheumatoid; biological therapy; epidemiology; spondylitis, ankylosing.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: JG has acted in an advisory role to many pharmaceutical companies (listed below), including to provide specific advice on malignancy risk with JAK inhibition for the purpose of regulatory review. He received no funding for this systematic review and meta-analysis, and there was no industry involvement or oversight for any stage of this project. JG has received honoraria from AbbVie, Biovitrum, BMS, Celgene, Chugai, Galapagos, Gilead, Janssen, Lilly, Novartis, Pfizer, Roche, Sanofi, Sobi and UCB. MDR has received honoraria from Lilly, Galapagos, Biogen and Menarini, and support for attending meetings from Lilly, Pfizer, Janssen and UCB. APC has received grant funding from BMS, and consulting fees from BMS, AbbVie and GSK/Galvini.
Figures


References
-
- Fleischmann R, Pangan AL, Song I-H, et al. . Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind, randomized controlled trial. Arthritis Rheumatol 2019;71:1788–800. 10.1002/art.41032 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

