Astrocyte reactivity influences amyloid-β effects on tau pathology in preclinical Alzheimer's disease
- PMID: 37248300
- PMCID: PMC10353939
- DOI: 10.1038/s41591-023-02380-x
Astrocyte reactivity influences amyloid-β effects on tau pathology in preclinical Alzheimer's disease
Abstract
An unresolved question for the understanding of Alzheimer's disease (AD) pathophysiology is why a significant percentage of amyloid-β (Aβ)-positive cognitively unimpaired (CU) individuals do not develop detectable downstream tau pathology and, consequently, clinical deterioration. In vitro evidence suggests that reactive astrocytes unleash Aβ effects in pathological tau phosphorylation. Here, in a biomarker study across three cohorts (n = 1,016), we tested whether astrocyte reactivity modulates the association of Aβ with tau phosphorylation in CU individuals. We found that Aβ was associated with increased plasma phosphorylated tau only in individuals positive for astrocyte reactivity (Ast+). Cross-sectional and longitudinal tau-positron emission tomography analyses revealed an AD-like pattern of tau tangle accumulation as a function of Aβ only in CU Ast+ individuals. Our findings suggest astrocyte reactivity as an important upstream event linking Aβ with initial tau pathology, which may have implications for the biological definition of preclinical AD and for selecting CU individuals for clinical trials.
© 2023. The Author(s).
Conflict of interest statement
H.C.K. and G.T.-B. are employees and stockholders of Johnson & Johnson. H.Z. has served on scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics and Wave; has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen and Roche and is a cofounder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). K.B. has served as a consultant, at advisory boards, or at data monitoring committees for Abcam, Axon, Biogen, JOMDD/Shimadzu, Julius Clinical, Lilly, MagQu, Novartis, Prothena, Roche Diagnostics and Siemens Healthineers and is a cofounder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, all unrelated to the work presented in this paper. S.G. has served as a scientific advisor to Cerveau Therapeutics. E.R.Z. serves on the scientific advisory board of Next Innovative Therapeutics (Nintx). P.R.-N. has served on scientific advisory boards and/or as a consultant for Eisai, Novo Nordisk and Roche. N.J.A. has given lectures in symposia sponsored by Lilly and Quanterix. M.D.I. has received research funding from GE Healthcare and Avid Radiopharmaceuticals. R.C.T. serves as a consultant, advisor and/or on the medical advisory board of Happify Health, Astellas Pharma, Bayer and Hello Therapeutics. GE Healthcare holds a license agreement with the University of Pittsburgh based on the PiB PET technology described in this paper. W.E.K. is a co-inventor of PiB and, as such, has a financial interest in this license agreement. GE Healthcare provided no grant support for this study and had no role in the design or interpretation of results or preparation of this paper. The other authors declare no competing interests.
Figures


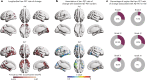
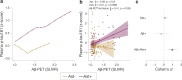

Update of
-
Astrocyte reactivity influences the association of amyloid-β and tau biomarkers in preclinical Alzheimer's disease.Res Sq [Preprint]. 2023 Feb 1:rs.3.rs-2507179. doi: 10.21203/rs.3.rs-2507179/v1. Res Sq. 2023. Update in: Nat Med. 2023 Jul;29(7):1775-1781. doi: 10.1038/s41591-023-02380-x. PMID: 36778243 Free PMC article. Updated. Preprint.
References
-
- Hansson O. Biomarkers for neurodegenerative diseases. Nat. Med. 2021;27:954–963. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG075336/AG/NIA NIH HHS/United States
- R01 AG052521/AG/NIA NIH HHS/United States
- R01 AG052528/AG/NIA NIH HHS/United States
- R37 AG023651/AG/NIA NIH HHS/United States
- R01 AG053504/AG/NIA NIH HHS/United States
- P30 AG066468/AG/NIA NIH HHS/United States
- R01 AG073267/AG/NIA NIH HHS/United States
- RF1 AG052525/AG/NIA NIH HHS/United States
- R01 HL105647/HL/NHLBI NIH HHS/United States
- P01 AG014449/AG/NIA NIH HHS/United States
- RF1 AG025516/AG/NIA NIH HHS/United States
- R01 AG052446/AG/NIA NIH HHS/United States
- RF1 AG053504/AG/NIA NIH HHS/United States
- K24 HL123565/HL/NHLBI NIH HHS/United States
- P01 AG025204/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

