A specific dispiropiperazine derivative that arrests cell cycle, induces apoptosis, necrosis and DNA damage
- PMID: 37248333
- PMCID: PMC10227070
- DOI: 10.1038/s41598-023-35927-6
A specific dispiropiperazine derivative that arrests cell cycle, induces apoptosis, necrosis and DNA damage
Abstract
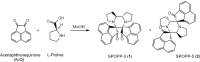
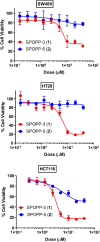
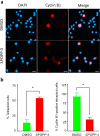
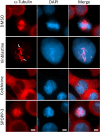
Dispiropiperazine compounds are a class of molecules known to confer biological activity, but those that have been studied as cell cycle regulators are few in number. Here, we report the characterization and synthesis of two dispiropiperazine derivatives: the previously synthesized spiro[2',3]-bis(acenaphthene-1'-one)perhydrodipyrrolo-[1,2-a:1,2-d]-pyrazine (SPOPP-3, 1), and its previously undescribed isomer, spiro[2',5']-bis(acenaphthene-1'-one)perhydrodipyrrolo-[1,2-a:1,2-d]-pyrazine (SPOPP-5, 2). SPOPP-3 (1), but not SPOPP-5 (2), was shown to have anti-proliferative activity against a panel of 18 human cancer cell lines with IC50 values ranging from 0.63 to 13 µM. Flow cytometry analysis revealed that SPOPP-3 (1) was able to arrest cell cycle at the G2/M phase in SW480 human cancer cells. Western blot analysis further confirmed the cell cycle arrest is in the M phase. In addition, SPOPP-3 (1) was shown to induce apoptosis, necrosis, and DNA damage as well as disrupt mitotic spindle positioning in SW480 cells. These results warrant further investigation of SPOPP-3 (1) as a novel anti-cancer agent, particularly for its potential ability to sensitize cancer cells for radiation-induced cell death, enhance cancer immunotherapy, overcome apoptosis-related drug resistance and for possible use in synthetic lethality cancer treatments.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Wang Y, et al. Radiosensitization by irinotecan is attributed to G2/M phase arrest, followed by enhanced apoptosis, probably through the ATM/Chk/Cdc25C/Cdc2 pathway in P53-mutant colorectal cancer cells. Int. J. Oncol. 2018;53:1667–1680. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

