Extrapolating empirical long-term survival data: the impact of updated follow-up data and parametric extrapolation methods on survival estimates in multiple myeloma
- PMID: 37248477
- PMCID: PMC10226243
- DOI: 10.1186/s12874-023-01952-2
Extrapolating empirical long-term survival data: the impact of updated follow-up data and parametric extrapolation methods on survival estimates in multiple myeloma
Abstract
Background: In economic evaluations, survival is often extrapolated to smooth out the Kaplan-Meier estimate and because the available data (e.g., from randomized controlled trials) are often right censored. Validation of the accuracy of extrapolated results can depend on the length of follow-up and the assumptions made about the survival hazard. Here, we analyze the accuracy of different extrapolation techniques while varying the data cut-off to estimate long-term survival in newly diagnosed multiple myeloma (MM) patients.
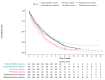
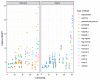
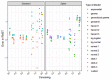
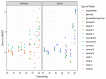
Methods: Empirical data were available from a randomized controlled trial and a registry for MM patients treated with melphalan + prednisone, thalidomide, and bortezomib- based regimens. Standard parametric and spline models were fitted while artificially reducing follow-up by introducing database locks. The maximum follow-up for these locks varied from 3 to 13 years. Extrapolated (conditional) restricted mean survival time (RMST) was compared to the Kaplan-Meier RMST and models were selected according to statistical tests, and visual fit.
Results: For all treatments, the RMST error decreased when follow-up and the absolute number of events increased, and censoring decreased. The decline in RMST error was highest when maximum follow-up exceeded six years. However, even when censoring is low there can still be considerable deviations in the extrapolated RMST conditional on survival until extrapolation when compared to the KM-estimate.
Conclusions: We demonstrate that both standard parametric and spline models could be worthy candidates when extrapolating survival for the populations examined. Nevertheless, researchers and decision makers should be wary of uncertainty in results even when censoring has decreased, and the number of events has increased.
Keywords: Kaplan-Meier; Multiple myeloma; Parametric Extrapolation; Survival.
© 2023. The Author(s).
Conflict of interest statement
FT reports previous consultation for AstraZeneca, Optimax Access, Dark Peak Analytics, and grants from Celgene outside the submitted work. Previous and ongoing research was or is partly funded by the CADTH (Canadian Agency for Drugs and Technologies in Health), the Dutch Ministry of Health, Welfare and Sport, and the European Haematology Association. HB reports previous research grants from BMS (Celgene BV), advisory board fee from Pfizer, outside the submitted work paid to the institute; Previous and ongoing research was or is partly funded by the CADTH (Canadian Agency for Drugs and Technologies in Health), the Dutch Healthcare Institute, and Medical Delta. LB reports previous and ongoing research grants from the European H2020 Research Programme and the Convergence Program outside the submitted work. WR reports previous and ongoing research grants from the European H2020 Research Programme and the Convergence Program outside the submitted work. CUG reports unrestricted grants from Boehringer Ingelheim, Astellas, Sanofi, Janssen-Cilag, Bayer, Sanofi, Amgen, Merck, Gilead, Novartis, and Astra Zeneca, Roche, and grants from European Research Programmes, CADTH (Canadian Agency for Drugs and Technologies in Health), the Dutch Healthcare Institute, the European Haematology Association, and Dutch Ministry of Health. All grants were outside the submitted work.
Figures
References
-
- Dutch Pharmacoeconomic Guidelines [Internet] Diemen: National Health Care Institute the Netherlands. Available from: https://www.zorginstituutnederland.nl/publicaties/publicatie/2016/02/29/....
-
- Latimer NR. Survival analysis for economic evaluations alongside clinical trials—extrapolation with patient-level data: inconsistencies, limitations, and a practical guide. Med Decis Making. 2013 Aug;33(6):743–54. - PubMed
-
- Everest L, Blommaert S, Chu RW, Chan KK, Parmar A. Parametric Survival Extrapolation of early Survival data in economic analyses: a comparison of projected Versus observed updated survival. Value in Health. 2021 Nov 24. - PubMed
-
- Mateos MV, Cavo M, Blade J, Dimopoulos MA, Suzuki K, Jakubowiak A, Knop S, Doyen C, Lucio P, Nagy Z, Pour L. Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): a randomised, open-label, phase 3 trial. The Lancet. 2020 Jan 11;395(10218):132 – 41.6. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

