Maintenance darunavir/ritonavir monotherapy to prevent perinatal HIV transmission, ANRS-MIE 168 MONOGEST study
- PMID: 37248782
- PMCID: PMC10320149
- DOI: 10.1093/jac/dkad161
Maintenance darunavir/ritonavir monotherapy to prevent perinatal HIV transmission, ANRS-MIE 168 MONOGEST study
Abstract
Objectives: Because NRTIs can have fetal toxicities, we evaluated a perinatal NRTI-sparing strategy to prevent perinatal HIV transmission. Our primary objective was to determine the proportion maintaining a viral load (VL) of <50 copies/mL up to delivery on darunavir/ritonavir monotherapy, without requiring treatment intensification.
Methods: In a one-arm, multicentre Phase 2 clinical trial, eligible patients in the first trimester of pregnancy on ART with plasma VL < 50 copies/mL received maintenance monotherapy with darunavir/ritonavir, 600/100 mg twice daily. VL was monitored monthly. ART was intensified in the case of VL > 50 copies/mL. Neonates received nevirapine prophylaxis for 14 days.
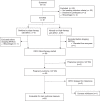
Results: Of 89 patients switching to darunavir/ritonavir monotherapy, 4 miscarried before 22 weeks' gestation, 2 changed treatment for elevated liver enzymes without virological failure, and 83 were evaluable for the main outcome. Six had virological failure confirmed on a repeat sample (median VL = 193 copies/mL; range 78-644), including two before switching to monotherapy. In these six cases, ART was intensified with tenofovir disoproxil fumarate/emtricitabine. The success rate was 75/83, 90.4% (95% CI, 81.9%-95.7%) considering two patients with VL missing at delivery as failures, and 77/83, 92.8% (95% CI, 84.9%-97.3%) when considering them as successes since both had undetectable VL on darunavir/ritonavir throughout pregnancy. In ITT, the last available VL before delivery was <50 copies/mL in all of the patients. There was no case of perinatal HIV transmission.
Conclusions: Darunavir/ritonavir maintenance monotherapy required intensification in nearly 10% of cases. This limits its widespread use, thus other regimens should be evaluated in order to limit exposure to antiretrovirals, particularly NRTIs, during pregnancy.
© The Author(s) 2023. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures
References
-
- EACS . Guidelines, Version 11.0, October 2021. 2021.https://www.eacsociety.org/media/final2021eacsguidelinesv11.0_oct2021.pdf.
-
- WHO . Update of Recommendations on First- and Second-line Antiretroviral Regimens. 2019.https://www.who.int/publications/i/item/WHO-CDS-HIV-19.15.
-
- Clinical Info HIV.gov . Recommendations for the Use of Antiretroviral Drugs in Pregnant Women with HIV Infection and Interventions to Reduce Perinatal HIV Transmission in the United States. https://clinicalinfo.hiv.gov/en/guidelines/perinatal/recommendations-arv....
-
- Morlat P. Prise en charge médicale des personnes vivant avec le VIH. Désir d’enfant et grossesse (May 2018). https://cns.sante.fr/wp-content/uploads/2017/11/experts-vih_grossesse.pdf.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical