Insulin signalling and GLUT4 trafficking in insulin resistance
- PMID: 37248992
- PMCID: PMC10317183
- DOI: 10.1042/BST20221066
Insulin signalling and GLUT4 trafficking in insulin resistance
Abstract
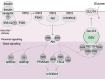
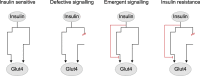
Insulin-stimulated glucose uptake into muscle and adipose tissue is vital for maintaining whole-body glucose homeostasis. Insulin promotes glucose uptake into these tissues by triggering a protein phosphorylation signalling cascade, which converges on multiple trafficking processes to deliver the glucose transporter GLUT4 to the cell surface. Impaired insulin-stimulated GLUT4 translocation in these tissues underlies insulin resistance, which is a major risk factor for type 2 diabetes and other metabolic diseases. Despite this, the precise changes in insulin signalling and GLUT4 trafficking underpinning insulin resistance remain unclear. In this review, we highlight insights from recent unbiased phosphoproteomics studies, which have enabled a comprehensive examination of insulin signalling and have transformed our perspective on how signalling changes may contribute to insulin resistance. We also discuss how GLUT4 trafficking is disrupted in insulin resistance, and underline sites where signalling changes could lead to these trafficking defects. Lastly, we address several major challenges currently faced by researchers in the field. As signalling and trafficking alterations can be examined at increasingly high resolution, integrative approaches examining the two in combination will provide immense opportunities for elucidating how they conspire to cause insulin resistance.
Keywords: GLUT4; glucose transport; insulin resistance; insulin signalling; phosphoproteomics; trafficking.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

Similar articles
-
GLUT4 Trafficking and Storage Vesicles: Molecular Architecture, Regulatory Networks, and Their Disruption in Insulin Resistance.Int J Mol Sci. 2025 Aug 5;26(15):7568. doi: 10.3390/ijms26157568. Int J Mol Sci. 2025. PMID: 40806697 Free PMC article. Review.
-
DOC2B promotes insulin sensitivity in mice via a novel KLC1-dependent mechanism in skeletal muscle.Diabetologia. 2019 May;62(5):845-859. doi: 10.1007/s00125-019-4824-2. Epub 2019 Feb 1. Diabetologia. 2019. PMID: 30707251 Free PMC article.
-
Mapping insulin/GLUT4 circuitry.Traffic. 2011 Jun;12(6):672-81. doi: 10.1111/j.1600-0854.2011.01178.x. Epub 2011 Mar 15. Traffic. 2011. PMID: 21401839 Review.
-
Microtubule-mediated GLUT4 trafficking is disrupted in insulin-resistant skeletal muscle.Elife. 2023 Apr 19;12:e83338. doi: 10.7554/eLife.83338. Elife. 2023. PMID: 37073948 Free PMC article.
-
Intracellular organization of insulin signaling and GLUT4 translocation.Recent Prog Horm Res. 2001;56:175-93. doi: 10.1210/rp.56.1.175. Recent Prog Horm Res. 2001. PMID: 11237212 Review.
Cited by
-
EFR3A, an Intriguing Gene, and Protein with a Scaffolding Function.Cells. 2025 Mar 17;14(6):445. doi: 10.3390/cells14060445. Cells. 2025. PMID: 40136694 Free PMC article. Review.
-
Hexokinase-linked glycolytic overload and unscheduled glycolysis in hyperglycemia-induced pathogenesis of insulin resistance, beta-cell glucotoxicity, and diabetic vascular complications.Front Endocrinol (Lausanne). 2024 Jan 16;14:1268308. doi: 10.3389/fendo.2023.1268308. eCollection 2023. Front Endocrinol (Lausanne). 2024. PMID: 38292764 Free PMC article.
-
Deciphering the role of classical oestrogen receptor in insulin resistance and type 2 diabetes mellitus: From molecular mechanism to clinical evidence.Bioimpacts. 2024 Aug 4;15:30378. doi: 10.34172/bi.30378. eCollection 2025. Bioimpacts. 2024. PMID: 40256228 Free PMC article. Review.
-
Protective Role of Dietary Polyphenols in the Management and Treatment of Type 2 Diabetes Mellitus.Nutrients. 2025 Jan 13;17(2):275. doi: 10.3390/nu17020275. Nutrients. 2025. PMID: 39861406 Free PMC article. Review.
-
SLC7 transporters at the crossroads of amino acid metabolism and diabetes pathophysiology: insights and therapeutic perspectives.Front Nutr. 2025 May 21;12:1467057. doi: 10.3389/fnut.2025.1467057. eCollection 2025. Front Nutr. 2025. PMID: 40469683 Free PMC article. Review.
References
-
- Rothman, D.L., Magnusson, I., Cline, G., Gerard, D., Kahn, C.R., Shulman, R.G.et al. (1995) Decreased muscle glucose transport/phosphorylation is an early defect in the pathogenesis of non-insulin-dependent diabetes mellitus. Proc. Natl Acad. Sci. U.S.A. 92, 983–987 10.1073/pnas.92.4.983 - DOI - PMC - PubMed
-
- Garvey, W.T., Maianu, L., Zhu, J.H., Hancock, J.A. and Golichowski, A.M. (1993) Multiple defects in the adipocyte glucose transport system cause cellular insulin resistance in gestational diabetes. heterogeneity in the number and a novel abnormality in subcellular localization of GLUT4 glucose transporters. Diabetes 42, 1773–1785 10.2337/diabetes.42.12.1773 - DOI - PubMed
-
- Garvey, W.T., Maianu, L., Zhu, J.H., Brechtel-Hook, G., Wallace, P. and Baron, A.D. (1998) Evidence for defects in the trafficking and translocation of GLUT4 glucose transporters in skeletal muscle as a cause of human insulin resistance. J. Clin. Invest. 101, 2377–2386 10.1172/JCI1557 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

