Nondomain biopolymers: Flexible molecular strategies to acquire biological functions
- PMID: 37249032
- PMCID: PMC11448004
- DOI: 10.1111/gtc.13050
Nondomain biopolymers: Flexible molecular strategies to acquire biological functions
Abstract
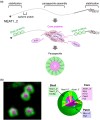
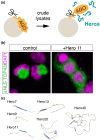
A long-standing assumption in molecular biology posits that the conservation of protein and nucleic acid sequences emphasizes the functional significance of biomolecules. These conserved sequences fold into distinct secondary and tertiary structures, enable highly specific molecular interactions, and regulate complex yet organized molecular processes within living cells. However, recent evidence suggests that biomolecules can also function through primary sequence regions that lack conservation across species or gene families. These regions typically do not form rigid structures, and their inherent flexibility is critical for their functional roles. This review examines the emerging roles and molecular mechanisms of "nondomain biomolecules," whose functions are not easily predicted due to the absence of conserved functional domains. We propose the hypothesis that both domain- and nondomain-type molecules work together to enable flexible and efficient molecular processes within the highly crowded intracellular environment.
Keywords: intrinsically disordered proteins; noncoding RNA; nondomain biopolymers; nonmembranous organelles; nuclear bodies; phase separation.
© 2023 The Authors. Genes to Cells published by Molecular Biology Society of Japan and John Wiley & Sons Australia, Ltd.
Figures




References
-
- Adams, M. D. , Celniker, S. E. , Holt, R. A. , Evans, C. A. , Gocayne, J. D. , Amanatides, P. G. , Scherer, S. E. , Li, P. W. , Hoskins, R. A. , Galle, R. F. , George, R. A. , Lewis, S. E. , Richards, S. , Ashburner, M. , Henderson, S. N. , Sutton, G. G. , Wortman, J. R. , Yandell, M. D. , Zhang, Q. , … Venter, J. C. (2000). The genome sequence of Drosophila melanogaster . Science, 287, 2185–2195. - PubMed
-
- Anger, A. M. , Armache, J. P. , Berninghausen, O. , Habeck, M. , Subklewe, M. , Wilson, D. N. , & Beckmann, R. (2013). Structures of the human and Drosophila 80S ribosome. Nature, 497, 80–85. - PubMed
-
- Arakawa, K. (2022). Examples of extreme survival: Tardigrade genomics and molecular Anhydrobiology. Annual Review of Animal Biosciences, 10, 17–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 21H05273/Ministry of Education, Culture, Sports, Science and Technology
- 21H05274/Ministry of Education, Culture, Sports, Science and Technology
- 21H05275/Ministry of Education, Culture, Sports, Science and Technology
- 21H05276/Ministry of Education, Culture, Sports, Science and Technology
- 21H05277/Ministry of Education, Culture, Sports, Science and Technology
- 21H05278/Ministry of Education, Culture, Sports, Science and Technology
- 21H05279/Ministry of Education, Culture, Sports, Science and Technology
- 21H05280/Ministry of Education, Culture, Sports, Science and Technology
- 21H05281/Ministry of Education, Culture, Sports, Science and Technology
- 21H05282/Ministry of Education, Culture, Sports, Science and Technology
LinkOut - more resources
Full Text Sources

