Cardiovascular complications in chronic kidney disease: a review from the European Renal and Cardiovascular Medicine Working Group of the European Renal Association
- PMID: 37249051
- PMCID: PMC10478756
- DOI: 10.1093/cvr/cvad083
Cardiovascular complications in chronic kidney disease: a review from the European Renal and Cardiovascular Medicine Working Group of the European Renal Association
Abstract
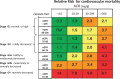
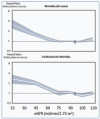
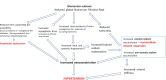
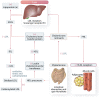
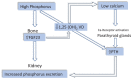
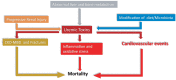
Chronic kidney disease (CKD) is classified into five stages with kidney failure being the most severe stage (stage G5). CKD conveys a high risk for coronary artery disease, heart failure, arrhythmias, and sudden cardiac death. Cardiovascular complications are the most common causes of death in patients with kidney failure (stage G5) who are maintained on regular dialysis treatment. Because of the high death rate attributable to cardiovascular (CV) disease, most patients with progressive CKD die before reaching kidney failure. Classical risk factors implicated in CV disease are involved in the early stages of CKD. In intermediate and late stages, non-traditional risk factors, including iso-osmotic and non-osmotic sodium retention, volume expansion, anaemia, inflammation, malnutrition, sympathetic overactivity, mineral bone disorders, accumulation of a class of endogenous compounds called 'uremic toxins', and a variety of hormonal disorders are the main factors that accelerate the progression of CV disease in these patients. Arterial disease in CKD patients is characterized by an almost unique propensity to calcification and vascular stiffness. Left ventricular hypertrophy, a major risk factor for heart failure, occurs early in CKD and reaches a prevalence of 70-80% in patients with kidney failure. Recent clinical trials have shown the potential benefits of hypoxia-inducible factor prolyl hydroxylase inhibitors, especially as an oral agent in CKD patients. Likewise, the value of proactively administered intravenous iron for safely treating anaemia in dialysis patients has been shown. Sodium/glucose cotransporter-2 inhibitors are now fully emerged as a class of drugs that substantially reduces the risk for CV complications in patients who are already being treated with adequate doses of inhibitors of the renin-angiotensin system. Concerted efforts are being made by major scientific societies to advance basic and clinical research on CV disease in patients with CKD, a research area that remains insufficiently explored.
Keywords: Cardiovascular disease; Chronic kidney disease; Clinical aspects; Death; Heart failure; Sudden death.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: None declared.
Figures

References
-
- Cockwell P, Fisher L-A. The global burden of chronic kidney disease. Lancet 2020;395:662–664. - PubMed
-
- Jager KJ, Kovesdy C, Langham R, Rosenberg M, Jha V, Zoccali C. A single number for advocacy and communication—worldwide more than 850 million individuals have kidney diseases. Kidney Int 2019;96:1048–1050. - PubMed
-
- Xie Y, Bowe B, Mokdad AH, Xian H, Yan Y, Li T, Maddukuri G, Tsai C-Y, Floyd T, Al-Aly Z. Analysis of the global burden of disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int 2018;94:567–581. - PubMed
-
- Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M, Pletcher MA, Smith AE, Tang K, Yuan CW, Brown JC, Friedman J, He J, Heuton KR, Holmberg M, Patel DJ, Reidy P, Carter A, Cercy K, Chapin A, Douwes-Schultz D, Frank T, Goettsch F, Liu PY, Nandakumar V, Reitsma MB, Reuter V, Sadat N, Sorensen RJD, Srinivasan V, Updike RL, York H, Lopez AD, Lozano R, Lim SS, Mokdad AH, Vollset SE, Murray CJL. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet 2018;392:2052–2090. - PMC - PubMed

