The Bulk Breast Cancer Cell and Breast Cancer Stem Cell Activity of Binuclear Copper(II)-Phenanthroline Complexes
- PMID: 37249243
- PMCID: PMC10947161
- DOI: 10.1002/chem.202301188
The Bulk Breast Cancer Cell and Breast Cancer Stem Cell Activity of Binuclear Copper(II)-Phenanthroline Complexes
Abstract
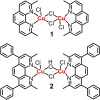
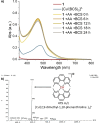
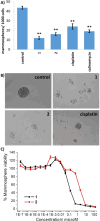
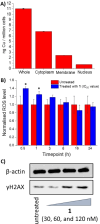
Mononuclear copper(II)-phenanthroline complexes have been widely investigated as anticancer agents whereas multinuclear copper(II)-phenanthroline complexes are underexplored. Here the synthesis and characterisation of two new binuclear copper(II)-phenanthroline complexes 1 and 2 is reported, comprising of 2,9-dimethyl-1,10-phenanthroline or 2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline, terminal chloride ligands, and bridging chloride or hydroxide ligands. The binuclear copper(II) complex containing 2,9-dimethyl-1,10-phenanthroline 1 displays nanomolar toxicity towards bulk breast cancer cells and breast cancer stem cells (CSCs) grown in monolayers, >50-fold greater than cisplatin (an anticancer metallodrug) and salinomycin (a gold-standard anti-CSC agent). Spectacularly, 1 exhibits >100-fold greater potency toward three-dimensionally cultured mammospheres than cisplatin and salinomycin. Mechanistic studies show that 1 evokes breast CSC apoptosis by elevating intracellular reactive oxygen species levels and damaging genomic DNA (possibly by an oxidative mechanism). To the best of our knowledge, this is the first study to probe the anti-breast CSC properties of binuclear copper(II)-phenanthroline complexes.
Keywords: antitumour agent; artificial metallonuclease; bioinorganic chemistry; cancer stem cell; copper.
© 2023 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Sung H., Ferlay J., Siegel R. L., Laversanne M., Soerjomataram I., Jemal A., Bray F., Ca-Cancer J. Clin. 2021, 71, 209–249. - PubMed
-
- None
-
- Lebwohl D., Canetta R., Eur. J. Cancer 1998, 34, 1522–1534; - PubMed
-
- Zhu J., Chen Z., Lallemand-Breitenbach V., de The H., Nat. Rev. Cancer 2002, 2, 705–713. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

