Hypermetabolism in mice carrying a near-complete human chromosome 21
- PMID: 37249575
- PMCID: PMC10229126
- DOI: 10.7554/eLife.86023
Hypermetabolism in mice carrying a near-complete human chromosome 21
Abstract
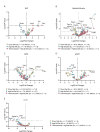
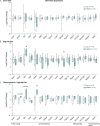
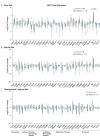
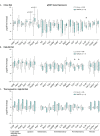
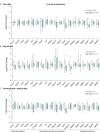
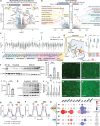
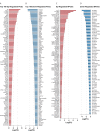
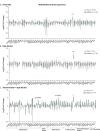
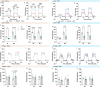
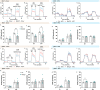
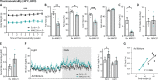
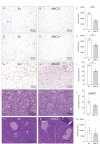
The consequences of aneuploidy have traditionally been studied in cell and animal models in which the extrachromosomal DNA is from the same species. Here, we explore a fundamental question concerning the impact of aneuploidy on systemic metabolism using a non-mosaic transchromosomic mouse model (TcMAC21) carrying a near-complete human chromosome 21. Independent of diets and housing temperatures, TcMAC21 mice consume more calories, are hyperactive and hypermetabolic, remain consistently lean and profoundly insulin sensitive, and have a higher body temperature. The hypermetabolism and elevated thermogenesis are likely due to a combination of increased activity level and sarcolipin overexpression in the skeletal muscle, resulting in futile sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) activity and energy dissipation. Mitochondrial respiration is also markedly increased in skeletal muscle to meet the high ATP demand created by the futile cycle and hyperactivity. This serendipitous discovery provides proof-of-concept that sarcolipin-mediated thermogenesis via uncoupling of the SERCA pump can be harnessed to promote energy expenditure and metabolic health.
Keywords: SERCA pump; aneuploidy; chromosomes; futile cycle; gene expression; hypermetabolism; mouse; sarcolipin; trisomy.
© 2023, Sarver et al.
Conflict of interest statement
DS, CX, SR, SA, AJ, FG, MD, MP, YK, RR, GW No competing interests declared, MO M.O. is CEO, employee, and shareholder of Trans Chromosomics, Inc which manages commercial use of the TcMAC21 mouse. We declare that none of the authors has a conflict of interest
Figures











Update of
-
Hypermetabolism in mice carrying a near complete human chromosome 21.bioRxiv [Preprint]. 2023 Jan 31:2023.01.30.526183. doi: 10.1101/2023.01.30.526183. bioRxiv. 2023. Update in: Elife. 2023 May 30;12:e86023. doi: 10.7554/eLife.86023. PMID: 36778465 Free PMC article. Updated. Preprint.
Similar articles
-
Hypermetabolism in mice carrying a near complete human chromosome 21.bioRxiv [Preprint]. 2023 Jan 31:2023.01.30.526183. doi: 10.1101/2023.01.30.526183. bioRxiv. 2023. Update in: Elife. 2023 May 30;12:e86023. doi: 10.7554/eLife.86023. PMID: 36778465 Free PMC article. Updated. Preprint.
-
Sarcolipin protein interaction with sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) is distinct from phospholamban protein, and only sarcolipin can promote uncoupling of the SERCA pump.J Biol Chem. 2013 Mar 8;288(10):6881-9. doi: 10.1074/jbc.M112.436915. Epub 2013 Jan 22. J Biol Chem. 2013. PMID: 23341466 Free PMC article.
-
Sarcolipin: A Key Thermogenic and Metabolic Regulator in Skeletal Muscle.Trends Endocrinol Metab. 2016 Dec;27(12):881-892. doi: 10.1016/j.tem.2016.08.006. Epub 2016 Sep 13. Trends Endocrinol Metab. 2016. PMID: 27637585 Free PMC article. Review.
-
The effects of sarcolipin over-expression in mouse skeletal muscle on metabolic activity.Arch Biochem Biophys. 2015 Mar 1;569:26-31. doi: 10.1016/j.abb.2015.01.027. Epub 2015 Feb 7. Arch Biochem Biophys. 2015. PMID: 25660043 Free PMC article.
-
Phospholamban and sarcolipin: Are they functionally redundant or distinct regulators of the Sarco(Endo)Plasmic Reticulum Calcium ATPase?J Mol Cell Cardiol. 2016 Feb;91:81-91. doi: 10.1016/j.yjmcc.2015.12.030. Epub 2015 Dec 29. J Mol Cell Cardiol. 2016. PMID: 26743715 Free PMC article. Review.
Cited by
-
TcMAC21 mouse model recapitulates abnormal vascular physiology observed in humans with Down syndrome.Physiol Rep. 2025 Jun;13(11):e70384. doi: 10.14814/phy2.70384. Physiol Rep. 2025. PMID: 40474784 Free PMC article.
-
Mediator kinase inhibition suppresses hyperactive interferon signaling in Down syndrome.Elife. 2025 Feb 10;13:RP100197. doi: 10.7554/eLife.100197. Elife. 2025. PMID: 39928031 Free PMC article.
-
The Role of lncRNAs in Pig Muscle in Response to Cold Exposure.Genes (Basel). 2023 Sep 30;14(10):1901. doi: 10.3390/genes14101901. Genes (Basel). 2023. PMID: 37895249 Free PMC article.
-
Patterns of Aneuploidy and Signaling Consequences in Cancer.Cancer Res. 2024 Aug 15;84(16):2575-2587. doi: 10.1158/0008-5472.CAN-24-0169. Cancer Res. 2024. PMID: 38924459 Free PMC article. Review.
-
Trisomic rescue via allele-specific multiple chromosome cleavage using CRISPR-Cas9 in trisomy 21 cells.PNAS Nexus. 2025 Feb 18;4(2):pgaf022. doi: 10.1093/pnasnexus/pgaf022. eCollection 2025 Feb. PNAS Nexus. 2025. PMID: 39967679 Free PMC article.
References
-
- Acin-Perez R, Benador IY, Petcherski A, Veliova M, Benavides GA, Lagarrigue S, Caudal A, Vergnes L, Murphy AN, Karamanlidis G, Tian R, Reue K, Wanagat J, Sacks H, Amati F, Darley-Usmar VM, Liesa M, Divakaruni AS, Stiles L, Shirihai OS. A novel approach to measure mitochondrial respiration in frozen biological samples. The EMBO Journal. 2020;39:e104073. doi: 10.15252/embj.2019104073. - DOI - PMC - PubMed
-
- Bal NC, Maurya SK, Sopariwala DH, Sahoo SK, Gupta SC, Shaikh SA, Pant M, Rowland LA, Bombardier E, Goonasekera SA, Tupling AR, Molkentin JD, Periasamy M. Sarcolipin is a newly identified regulator of muscle-based Thermogenesis in mammals. Nature Medicine. 2012;18:1575–1579. doi: 10.1038/nm.2897. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

