The Intramolecular Povarov Tool in the Construction of Fused Nitrogen-Containing Heterocycles
- PMID: 37249641
- PMCID: PMC10229753
- DOI: 10.1007/s41061-023-00428-7
The Intramolecular Povarov Tool in the Construction of Fused Nitrogen-Containing Heterocycles
Abstract
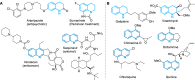
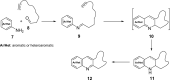
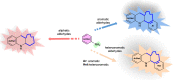
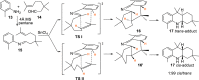
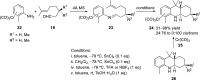
Nitrogen heterocycles are part of the structure of natural products and agents with important biological activity, such as antiviral, antibiotic, and antitumor drugs. For this reason, heterocyclic compounds are one of today's most desirable synthetic targets and the Povarov reaction is a powerful synthetic tool for the construction of highly functionalized heterocyclic systems. This process involves an aromatic amine, a carbonyl compound, and an olefin or acetylene to give rise to the formation of a nitrogen-containing heterocycle. This review illustrates advances in the synthetic aspects of the intramolecular Povarov reaction for the construction of intricate nitrogen-containing polyheterocyclic compounds. This original review presents research done in this field, with references to important works by internationally relevant research groups on this current topic, covering the literature from 1992 to 2022. The intramolecular Povarov reactions are described here according to the key processes involved, using different combinations of aromatic or heteroaromatic amines, and aliphatic, aromatic, or heteroaromatic aldehydes. Some catalytic reactions promoted by transition metals are detailed, as well as the oxidative Povarov reaction and some asymmetric intramolecular Povarov processes.
Keywords: Fused nitrogenated heterocycles; Intramolecular; Povarov reaction; [4 + 2]-cycloaddition.
© 2023. The Author(s).
Conflict of interest statement
The authors do not have any conflicts of interest to declare.
Figures
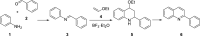




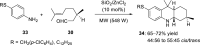
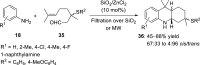
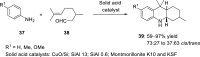
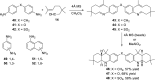


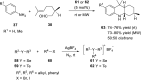
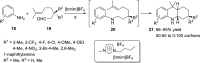
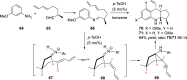
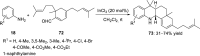
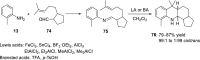
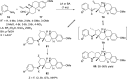
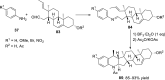

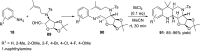

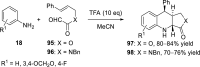

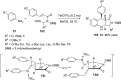

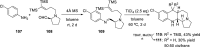
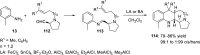
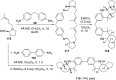
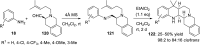
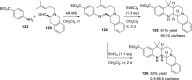
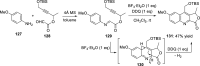
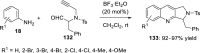

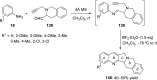
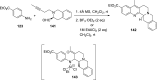
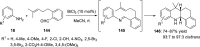
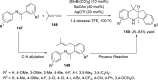
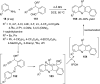
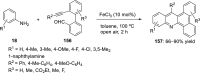
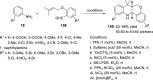
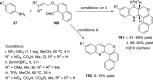
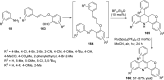
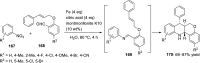

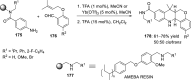

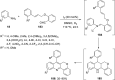
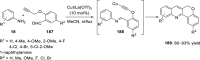


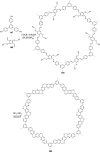

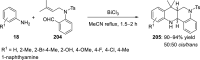
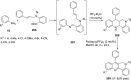
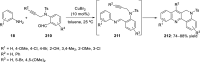


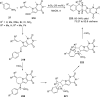
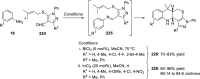
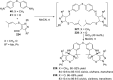
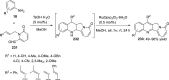

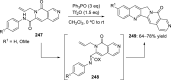
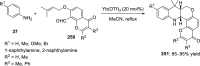
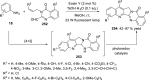
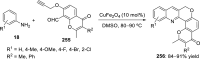
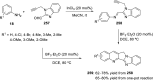
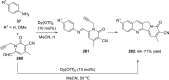



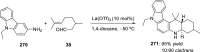
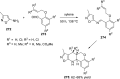
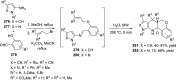
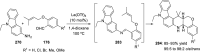

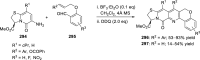
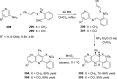
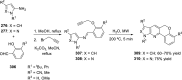
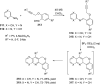


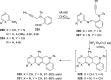

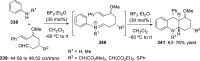
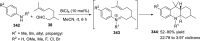

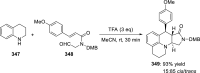
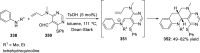
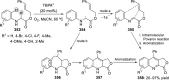
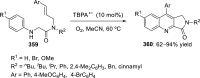

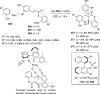
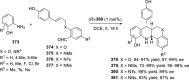


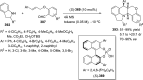
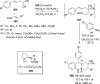
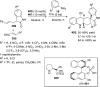
References
-
- Liu J, Jiang J, Zheng L, Liu ZQ. Recent advances in the synthesis of nitrogen heterocycles using arenediazonium salts as nitrogen sources. Adv Synth Catal. 2020;362:4876–4895. doi: 10.1002/adsc.202000700. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
