Therapeutic inhibition of CXCR1/2: where do we stand?
- PMID: 37249756
- PMCID: PMC10227827
- DOI: 10.1007/s11739-023-03309-5
Therapeutic inhibition of CXCR1/2: where do we stand?
Abstract
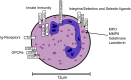
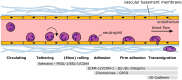
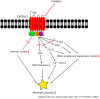
Mounting experimental evidence from in vitro and in vivo animal studies points to an essential role of the CXCL8-CXCR1/2 axis in neutrophils in the pathophysiology of inflammatory and autoimmune diseases. In addition, the pathogenetic involvement of neutrophils and the CXCL8-CXCR1/2 axis in cancer progression and metastasis is increasingly recognized. Consequently, therapeutic targeting of CXCR1/2 or CXCL8 has been intensively investigated in recent years using a wide array of in vitro and animal disease models. While a significant benefit for patients with unwanted neutrophil-mediated inflammatory conditions may be expected from a potential clinical use of inhibitors, their use in severe infections or sepsis might be problematic and should be carefully and thoroughly evaluated in animal models and clinical trials. Translating the approaches using inhibitors of the CXCL8-CXCR1/2 axis to cancer therapy is definitively a new and promising research avenue, which parallels the ongoing efforts to clearly define the involvement of neutrophils and the CXCL8-CXCR1/2 axis in neoplastic diseases. Our narrative review summarizes the current literature on the activation and inhibition of these receptors in neutrophils, key inhibitor classes for CXCR2 and the therapeutic relevance of CXCR2 inhibition focusing here on gastrointestinal diseases.
Keywords: CXCR2; Cancer; Chemokine receptor inhibition; Gut; Inflammation; Neutrophils.
© 2023. The Author(s), under exclusive licence to Società Italiana di Medicina Interna (SIMI).
Conflict of interest statement
MS received support by a project grant from Dompè Farmaceutici S.p.A. RB is a preclinical pharmacology consultant of Dompé Farmaceutici S.p.a.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

