What we talk about when we talk about COVID-19 vaccination campaign impact: a narrative review
- PMID: 37250083
- PMCID: PMC10211334
- DOI: 10.3389/fpubh.2023.1126461
What we talk about when we talk about COVID-19 vaccination campaign impact: a narrative review
Abstract
Background: The lack of precise definitions and terminological consensus about the impact studies of COVID-19 vaccination leads to confusing statements from the scientific community about what a vaccination impact study is.
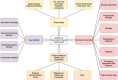
Objective: The present work presents a narrative review, describing and discussing COVID-19 vaccination impact studies, mapping their relevant characteristics, such as study design, approaches and outcome variables, while analyzing their similarities, distinctions, and main insights.
Methods: The articles screening, regarding title, abstract, and full-text reading, included papers addressing perspectives about the impact of vaccines on population outcomes. The screening process included articles published before June 10, 2022, based on the initial papers' relevance to this study's research topics. The main inclusion criteria were data analyses and study designs based on statistical modelling or comparison of pre- and post-vaccination population.
Results: The review included 18 studies evaluating the vaccine impact in a total of 48 countries, including 32 high-income countries (United States, Israel, and 30 Western European countries) and 16 low- and middle-income countries (Brazil, Colombia, and 14 Eastern European countries). We summarize the main characteristics of the vaccination impact studies analyzed in this narrative review.
Conclusion: Although all studies claim to address the impact of a vaccination program, they differ significantly in their objectives since they adopt different definitions of impact, methodologies, and outcome variables. These and other differences are related to distinct data sources, designs, analysis methods, models, and approaches.
Keywords: COVID-19; narrative review; populational studies; vaccination campaign; vaccine impact.
Copyright © 2023 Hastenreiter Filho, Peres, Maddalena, Baião, Ranzani, Hamacher, Maçaira and Bozza.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Hunter PR, Brainard J. (2021). Estimating the effectiveness of the Pfizer COVID-19 BNT162b2 vaccine after a single dose. A reanalysis of a study of ‘real-world’ vaccination outcomes from Israel. medRxiv [Preprint]. doi: 10.1101/2021.02.01.21250957
-
- World health Organization (2021). Evaluation of COVID-19 vaccine effectiveness: interim guidance. Available at: https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-vaccine_e... (Accessed April 11, 2022).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

