Recent Advances in Pyridine Scaffold: Focus on Chemistry, Synthesis, and Antibacterial Activities
- PMID: 37250749
- PMCID: PMC10212683
- DOI: 10.1155/2023/9967591
Recent Advances in Pyridine Scaffold: Focus on Chemistry, Synthesis, and Antibacterial Activities
Abstract
Multidrug-resistant (MDR) pathogens have created a fatal problem for human health and antimicrobial treatment. Among the currently available antibiotics, many are inactive against MDR pathogens. In this context, heterocyclic compounds/drugs play a vital role. Thus, it is very much essential to explore new research to combat the issue. Of the available nitrogen-bearing heterocyclic compounds/drugs, pyridine derivatives are of special interest due to their solubility. Encouragingly, some of the newly synthesized pyridine compounds/drugs are found to inhibit multidrug-resistant S. aureus (MRSA). Pyridine scaffold bearing poor basicity generally improves water solubility in pharmaceutically potential molecules and has led to the discovery of numerous broad-spectrum therapeutic agents. Keeping these in mind, we have reviewed the chemistry, recent synthetic techniques, and bacterial preventative activity of pyridine derivatives since 2015. This will facilitate the development of pyridine-based novel antibiotic/drug design in the near future as a versatile scaffold with limited side effects for the next-generation therapeutics.
Copyright © 2023 Md. Badrul Islam et al.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



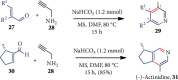


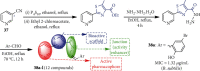
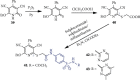
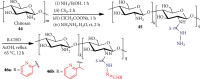
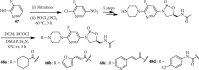
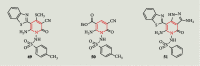

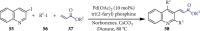
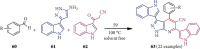





References
-
- Dhavale D. D., Matin M. M. Selective sulfonylation of 4- C-hydroxymethyl-β-l-threo-pento-1,4-furanose: synthesis of bicyclic diazasugars. Tetrahedron . 2004;60(19):4275–4281. doi: 10.1016/j.tet.2004.03.034. - DOI
-
- Albratty M., Alhazmi H. A. Novel pyridine and pyrimidine derivatives as promising anticancer agents: a review. Arabian Journal of Chemistry . 2022;15(6, article 103846) doi: 10.1016/j.arabjc.2022.103846. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

