Overview of cellular homeostasis-associated nuclear envelope lamins and associated input signals
- PMID: 37250905
- PMCID: PMC10213260
- DOI: 10.3389/fcell.2023.1173514
Overview of cellular homeostasis-associated nuclear envelope lamins and associated input signals
Abstract
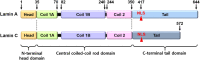
With the discovery of the role of the nuclear envelope protein lamin in human genetic diseases, further diverse roles of lamins have been elucidated. The roles of lamins have been addressed in cellular homeostasis including gene regulation, cell cycle, cellular senescence, adipogenesis, bone remodeling as well as modulation of cancer biology. Features of laminopathies line with oxidative stress-associated cellular senescence, differentiation, and longevity and share with downstream of aging-oxidative stress. Thus, in this review, we highlighted various roles of lamin as key molecule of nuclear maintenance, specially lamin-A/C, and mutated LMNA gene clearly reveal aging-related genetic phenotypes, such as enhanced differentiation, adipogenesis, and osteoporosis. The modulatory roles of lamin-A/C in stem cell differentiation, skin, cardiac regulation, and oncology have also been elucidated. In addition to recent advances in laminopathies, we highlighted for the first kinase-dependent nuclear lamin biology and recently developed modulatory mechanisms or effector signals of lamin regulation. Advanced knowledge of the lamin-A/C proteins as diverse signaling modulators might be biological key to unlocking the complex signaling of aging-related human diseases and homeostasis in cellular process.
Keywords: cell cycle; lamin; nuclear envelope; redox hemostasis; scenescence.
Copyright © 2023 Kim, Lee and Hong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Afonso P., Auclair M., Boccara F., Vantyghem M. C., Katlama C., Capeau J., et al. (2016). LMNA mutations resulting in lipodystrophy and HIV protease inhibitors trigger vascular smooth muscle cell senescence and calcification: Role of ZMPSTE24 downregulation. Atherosclerosis 245, 200–211. 10.1016/j.atherosclerosis.2015.12.012 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

