Synaptic properties of mouse tecto-parabigeminal pathways
- PMID: 37251004
- PMCID: PMC10213440
- DOI: 10.3389/fnsys.2023.1181052
Synaptic properties of mouse tecto-parabigeminal pathways
Abstract
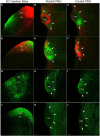
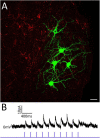
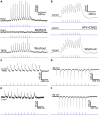
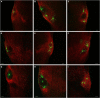
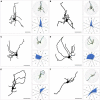
The superior colliculus (SC) is a critical hub for the generation of visually-evoked orienting and defensive behaviors. Among the SC's myriad downstream targets is the parabigeminal nucleus (PBG), the mammalian homolog of the nucleus isthmi, which has been implicated in motion processing and the production of defensive behaviors. The inputs to the PBG are thought to arise exclusively from the SC but little is known regarding the precise synaptic relationships linking the SC to the PBG. In the current study, we use optogenetics as well as viral tracing and electron microscopy in mice to better characterize the anatomical and functional properties of the SC-PBG circuit, as well as the morphological and ultrastructural characteristics of neurons residing in the PBG. We characterized GABAergic SC-PBG projections (that do not contain parvalbumin) and glutamatergic SC-PBG projections (which include neurons that contain parvalbumin). These two terminal populations were found to converge on different morphological populations of PBG neurons and elicit opposing postsynaptic effects. Additionally, we identified a population of non-tectal GABAergic terminals in the PBG that partially arise from neurons in the surrounding tegmentum, as well as several organizing principles that divide the nucleus into anatomically distinct regions and preserve a coarse retinotopy inherited from its SC-derived inputs. These studies provide an essential first step toward understanding how PBG circuits contribute to the initiation of behavior in response to visual signals.
Keywords: GABA; electron microscopy; nucleus isthmi; optogenetics; parabigeminal nucleus; parvalbumin; superior colliculus; tectum.
Copyright © 2023 Whyland, Masterson, Slusarczyk and Bickford.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

