Green-Engineered Barrier Creams with Montmorillonite-Chlorophyll Clays as Adsorbents for Benzene, Toluene, and Xylene
- PMID: 37251084
- PMCID: PMC10214870
- DOI: 10.3390/separations10040237
Green-Engineered Barrier Creams with Montmorillonite-Chlorophyll Clays as Adsorbents for Benzene, Toluene, and Xylene
Abstract
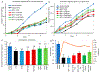
Dermal exposures to hazardous environmental chemicals in water can significantly affect the morphology and integrity of skin structure, leading to enhanced and deeper penetration. Organic solvents, such as benzene, toluene, and xylene (BTX), have been detected in humans following skin exposure. In this study, novel barrier cream formulations (EVB™) engineered with either montmorillonite (CM and SM) or chlorophyll-amended montmorillonite (CMCH and SMCH) clays were tested for their binding efficacy for BTX mixtures in water. The physicochemical properties of all sorbents and barrier creams were characterized and were shown to be suitable for topical application. In vitro adsorption results indicated that EVB-SMCH was the most effective and favorable barrier for BTX, as supported by the high binding percentage (29-59% at 0.05 g and 0.1 g), stable binding at equilibrium, low desorption rates, and high binding affinity. Pseudo-second-order and the Freundlich models best fit the adsorption kinetics and isotherms, and the adsorption was an exothermic reaction. Ecotoxicological models using L. minor and H. vulgaris that were submersed in aqueous culture media showed that the inclusion of 0.05% and 0.2% EVB-SMCH reduced BTX concentration. This result was further supported by the significant and dose-dependent increase in multiple growth endpoints, including plant frond number, surface area, chlorophyll content, growth rate, inhibition rate, and hydra morphology. The in vitro adsorption results and in vivo plant and animal models indicated that green-engineered EVB-SMCH can be used as an effective barrier to bind BTX mixtures and interrupt their diffusion and dermal contact.
Keywords: BTX; adsorption isotherm; barrier emulsion cream; bentonite clay; chlorophyll; dermal contact; ecotoxicological model; generally recognized as safe; kinetics; topical application.
Conflict of interest statement
Conflicts of Interest: The authors declare no conflict of interest.
Figures

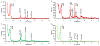
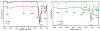
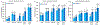
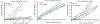


References
-
- Tursi A; Chidichimo F; Bagetta R; Beneduci A Btx removal from open aqueous systems by modified cellulose fibers and evaluation of competitive evaporation kinetics. Water 2020, 12, 3154.
-
- Namkung E; Rittmann BE Estimating volatile organic-compound emissions from publicly owned treatment works. J. Water Pollut. Control. Fed 1987, 59, 670–678.
-
- Wilbur S; Wohlers D; Paikoff S; Keith LS; Farron O Atsdr evaluation of potential for human exposure to benzene. Toxicol. Ind. Health 2008, 24, 399–442. - PubMed
-
- Mrowiec B Effect of btx on biological treatment of sewage. Environ. Prot. Eng 2009, 35, 197–206.
-
- van Afferden M; Rahman KZ; Mosig P; De Biase C; Thullner M; Oswald SE; Muller RA Remediation of groundwater contaminated with mtbe and benzene: The potential of vertical-flow soil filter systems. Water Res. 2011, 45, 5063–5074. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
