Novel Eco-friendly, One-Pot Method for the Synthesis of Kynurenic Acid Ethyl Esters
- PMID: 37251176
- PMCID: PMC10210203
- DOI: 10.1021/acsomega.3c01170
Novel Eco-friendly, One-Pot Method for the Synthesis of Kynurenic Acid Ethyl Esters
Abstract
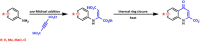
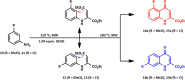
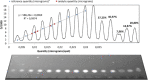
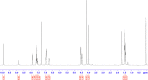
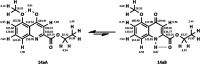
The synthesis of kynurenic acid derivatives with potential biological effect was investigated and optimized for one-batch, two-step microwave-assisted reactions. Utilizing both chemically and biologically representative non-, methyl-, methoxy-, and chlorosubstituted aniline derivatives, in catalyst-free conditions, syntheses of seven kynurenic acid derivatives were achieved in a time frame of 2-3.5 h. In place of halogenated reaction media, tuneable green solvents were introduced for each analogue. The potential of green solvent mixtures to replace traditional solvents and to alter the regioisomeric ratio regarding the Conrad-Limpach method was highlighted. The advantages of the fast, eco-friendly, inexpensive analytic technique of TLC densitometry were emphasized for reaction monitoring and conversion determination in comparison to quantitative NMR. Moreover, the developed 2-3.5 h syntheses were scaled-up to achieve gram-scale products of KYNA derivatives, without altering the reaction time in the halogenated solvent DCB and more importantly in its green substitutes.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
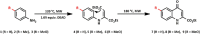


Similar articles
-
Convenient One-Pot Synthesis of Kynurenic Acid Ethyl Ester and Exploration to Direct Synthesis of Neuroprotective Kynurenic Acid and Amide Derivatives.J Org Chem. 2023 Aug 4;88(15):10494-10500. doi: 10.1021/acs.joc.3c00446. Epub 2023 Jul 18. J Org Chem. 2023. PMID: 37463064
-
Synthetic- and DFT modelling studies on regioselective modified Mannich reactions of hydroxy-KYNA derivatives.RSC Adv. 2020 Dec 24;11(1):543-554. doi: 10.1039/d0ra08325a. eCollection 2020 Dec 21. RSC Adv. 2020. PMID: 35423050 Free PMC article.
-
Green Synthesis of Fused Chromeno-pyrazolo-phthalazine Derivatives with Silicasupported Bismuth Nitrate under Solvent-free Conditions.Curr Org Synth. 2021;18(8):854-861. doi: 10.2174/1570179417666201208112520. Curr Org Synth. 2021. PMID: 33292122
-
Green Synthetic Approach: An Efficient Eco-Friendly Tool for Synthesis of Biologically Active Oxadiazole Derivatives.Molecules. 2021 Feb 22;26(4):1163. doi: 10.3390/molecules26041163. Molecules. 2021. PMID: 33671751 Free PMC article. Review.
-
Green solvents and technologies for oil extraction from oilseeds.Chem Cent J. 2017 Jan 23;11:9. doi: 10.1186/s13065-017-0238-8. eCollection 2017. Chem Cent J. 2017. PMID: 28123451 Free PMC article. Review.
Cited by
-
Selective heterofunctionalization of kynurenic acid derivatives.RSC Adv. 2025 Jul 23;15(32):26420-26427. doi: 10.1039/d5ra04301h. eCollection 2025 Jul 21. RSC Adv. 2025. PMID: 40703074 Free PMC article.
-
The Biology and Biochemistry of Kynurenic Acid, a Potential Nutraceutical with Multiple Biological Effects.Int J Mol Sci. 2024 Aug 21;25(16):9082. doi: 10.3390/ijms25169082. Int J Mol Sci. 2024. PMID: 39201768 Free PMC article. Review.
References
-
- Anastas P. T.; Warner J. C.. Green Chemistry: Theory and Practice; Oxford University Press: New York,, 1998.
-
- Byrne F. P.; Jin S.; Paggiola G.; Petchey T. H. M.; Clark J. H.; Farmer T. J.; Hunt A. J.; Robert McElroy C.; Sherwood J. Tools and Techniques for Solvent Selection: Green Solvent Selection Guides. Sustain Chem Process 2016, 4, 710.1186/s40508-016-0051-z. - DOI
-
- Capello C.; Fischer U.; Hungerbühler K. What Is a Green Solvent? A Comprehensive Framework for the Environmental Assessment of Solvents. Green Chem. 2007, 9, 927.10.1039/b617536h. - DOI
LinkOut - more resources
Full Text Sources

