Potential Repurposed Drug Candidates for Tuberculosis Treatment: Progress and Update of Drugs Identified in Over a Decade
- PMID: 37251185
- PMCID: PMC10210030
- DOI: 10.1021/acsomega.2c05511
Potential Repurposed Drug Candidates for Tuberculosis Treatment: Progress and Update of Drugs Identified in Over a Decade
Abstract
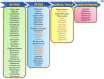
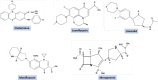
The devastating impact of Tuberculosis (TB) has been a menace to mankind for decades. The World Health Organization (WHO) End TB Strategy aims to reduce TB mortality up to 95% and 90% of overall TB cases worldwide, by 2035. This incessant urge will be achieved with a breakthrough in either a new TB vaccine or novel drugs with higher efficacy. However, the development of novel drugs is a laborious process involving a timeline of almost 20-30 years with huge expenditure; on the other hand, repurposing previously approved drugs is a viable technique for overcoming current bottlenecks in the identification of new anti-TB agents. The present comprehensive review discusses the progress of almost all the repurposed drugs that have been identified to the present day (∼100) and are in the development or clinical testing phase against TB. We have also emphasized the efficacy of repurposed drugs in combination with already available frontline anti-TB medications along with the scope of future investigations. This study would provide the researchers a detailed overview of nearly all identified anti-TB repurposed drugs and may assist them in selecting the lead compounds for further in vivo/clinical research.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
[Development of antituberculous drugs: current status and future prospects].Kekkaku. 2006 Dec;81(12):753-74. Kekkaku. 2006. PMID: 17240921 Review. Japanese.
-
Repurposed drug candidates for antituberculosis therapy.Eur J Med Chem. 2020 Apr 15;192:112175. doi: 10.1016/j.ejmech.2020.112175. Epub 2020 Feb 25. Eur J Med Chem. 2020. PMID: 32126450 Review.
-
[Recent progress in mycobacteriology].Kekkaku. 2007 Oct;82(10):783-99. Kekkaku. 2007. PMID: 18018602 Japanese.
-
[Frontier of mycobacterium research--host vs. mycobacterium].Kekkaku. 2005 Sep;80(9):613-29. Kekkaku. 2005. PMID: 16245793 Japanese.
-
Repurposed Drugs as Potential Therapeutic Candidates for the Management of Alzheimer's Disease.Curr Drug Metab. 2017;18(9):842-852. doi: 10.2174/1389200218666170607101622. Curr Drug Metab. 2017. PMID: 28595531 Review.
Cited by
-
AMMVF-DTI: A Novel Model Predicting Drug-Target Interactions Based on Attention Mechanism and Multi-View Fusion.Int J Mol Sci. 2023 Sep 15;24(18):14142. doi: 10.3390/ijms241814142. Int J Mol Sci. 2023. PMID: 37762445 Free PMC article.
-
A Comparative Pharmacokinetics Study of Orally and Intranasally Administered 8-Nitro-1,3-benzothiazin-4-one (BTZ043) Amorphous Drug Nanoparticles.ACS Pharmacol Transl Sci. 2024 Nov 9;7(12):4123-4134. doi: 10.1021/acsptsci.4c00558. eCollection 2024 Dec 13. ACS Pharmacol Transl Sci. 2024. PMID: 39698258
-
Overcoming Mycobacterium tuberculosis Drug Resistance: Novel Medications and Repositioning Strategies.ACS Omega. 2023 Sep 1;8(36):32244-32257. doi: 10.1021/acsomega.3c02563. eCollection 2023 Sep 12. ACS Omega. 2023. PMID: 37720746 Free PMC article. Review.
-
The structure of Mycobacterium thermoresistibile MmpS5 reveals a conserved disulfide bond across mycobacteria.Metallomics. 2024 Mar 12;16(3):mfae011. doi: 10.1093/mtomcs/mfae011. Metallomics. 2024. PMID: 38425033 Free PMC article.
-
Computational analysis of RNA methyltransferase Rv3366 as a potential drug target for combating drug-resistant Mycobacterium tuberculosis.Front Mol Biosci. 2024 Jan 11;10:1348337. doi: 10.3389/fmolb.2023.1348337. eCollection 2023. Front Mol Biosci. 2024. PMID: 38274093 Free PMC article.
References
-
- Global tuberculosis report 2021; World Health Organization; Geneva, 2021.
Publication types
LinkOut - more resources
Full Text Sources

