The manipulation of cell suspensions from zebrafish intestinal mucosa contributes to understanding enteritis
- PMID: 37251394
- PMCID: PMC10213505
- DOI: 10.3389/fimmu.2023.1193977
The manipulation of cell suspensions from zebrafish intestinal mucosa contributes to understanding enteritis
Abstract
Background: Although zebrafish are commonly used to study intestinal mucosal immunity, no dedicated procedure for isolating immune cells from zebrafish intestines is currently available. A speedy and simple operating approach for preparing cell suspension from mucosa has been devised to better understanding of intestinal cellular immunity in zebrafish.
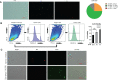
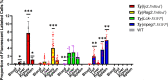
Methods and results: The mucosal villi were separated away from the muscle layer by repeated blows. The complete deprivation of mucosa was done and evidenced by HE and qPCR results. Higher expression of both innate (mpeg1, mpx, and lck) and adaptive immune genes (zap70, blnk, foxp3a, and foxp3b) was revealed compared to cells obtained by typical mesh rubbing. The cytometric results also revealed that the tested operation group had a higher concentration and viability. Further, fluorescent-labelled immune cells from 3mo Tg(lyz:DsRED2), Tg(mpeg1:EGFP), Tg(Rag2:DsRED), and Tg(lck:EGFP), were isolated and evaluated for the proportion, and immune cells' type could be inferred from the expression of marker genes. The transcriptomic data demonstrated that the intestinal immune cell suspension made using the new technique was enriched in immune-related genes and pathways, including il17a/f, il22, cd59, and zap70, as well as pattern recognition receptor signaling and cytokine-cytokine receptor interaction. In addition, the low expression of DEG for the adherent and close junctions indicated less muscular contamination. Also, lower expression of gel-forming mucus-associated genes in the mucosal cell suspension was consistent with the current less viscous cell suspension. To apply and validate the developed manipulation, enteritis was induced by soybean meal diet, and immune cell suspensions were analyzed by flow cytometry and qPCR. The finding that in enteritis samples, there was inflammatory increase of neutrophils and macrophages, was in line with upregulated cytokines (il8 and il10) and cell markers (mpeg1 and mpx).
Conclusion: As a result, the current work created a realistic technique for studying intestinal immune cells in zebrafish. The immune cells acquired may aid in further research and knowledge of intestinal illness at the cellular level.
Keywords: SBMIE; cell suspension; flow cytometry; intestine; transcriptome; zebrafish.
Copyright © 2023 Zhao, Liu, Xie, Zhang, Zhu, Su, Guo, Li, Wang, Zhang, Cheng, Wu and Xia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Venkatesh P, Jeyapriya SP, Suresh N, Vivekananthan T. Report on gut associated lymphoid tissue (GALT) in freshwater fish channa punctatus (Bloch). Int J Pure Appl Zool (2014) 2(2):95–9.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

