Ulipristal acetate versus levonorgestrel-releasing intrauterine system for heavy menstrual bleeding (UCON): a randomised controlled phase III trial
- PMID: 37251622
- PMCID: PMC10209678
- DOI: 10.1016/j.eclinm.2023.101995
Ulipristal acetate versus levonorgestrel-releasing intrauterine system for heavy menstrual bleeding (UCON): a randomised controlled phase III trial
Abstract
Background: Heavy menstrual bleeding affects one in four women and negatively impacts quality of life. Ulipristal acetate is prescribed to treat symptoms associated with uterine fibroids. We compared the effectiveness of ulipristal acetate and the levonorgestrel-releasing intrauterine system at reducing the burden of heavy menstrual bleeding, irrespective of the presence of fibroids.
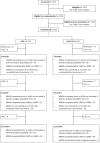
Methods: This randomised, open-label, parallel group phase III trial enrolled women over 18 years with heavy menstrual bleeding from 10 UK hospitals. Participants were centrally randomised, in a 1:1 ratio, to either three, 12-week treatment cycles of 5 mg ulipristal acetate daily, separated by 4-week treatment-free intervals, or a levonorgestrel-releasing intrauterine system. The primary outcome, analysed by intention-to-treat, was quality of life measured by the Menorrhagia Multi-Attribute Scale at 12 months. Secondary outcomes included menstrual bleeding and liver function. The trial is registered with ISRCTN, 20426843.
Findings: Between June 5th, 2015 and February 26th, 2020, 236 women were randomised, either side of a recruitment suspension due to concerns of ulipristal acetate hepatoxicity. Subsequent withdrawal of ulipristal acetate led to early cessation of recruitment but the trial continued in follow-up. The primary outcome substantially improved in both groups, and was 89, (interquartile range [IQR] 65 to 100, n = 53) and 94, (IQR 70 to 100, n = 50; adjusted odds ratio 0.55, 95% confidence interval [CI] 0.26-1.17; p = 0.12) in the ulipristal and levonorgestrel-releasing intrauterine system groups. Rates of amenorrhoea at 12 months were higher in those allocated ulipristal acetate compared to levonorgestrel-releasing intrauterine system (64% versus 25%, adjusted odds ratio 7.12, 95% CI 2.29-22.2). Other outcomes were similar between the two groups and there were no cases of endometrial malignancy or hepatotoxicity due to ulipristal acetate use.
Interpretation: Our findings suggested that both treatments improved quality of life. Ulipristal was more effective at inducing amenorrhoea. Ulipristal has been demonstrated to be an effective medical therapeutic option but currently its use has restrictions and requires liver function monitoring.
Funding: UK Medical Research Council and National Institute of Health Research EME Programme (12/206/52).
Keywords: Adenomyosis endometrium; Amenorrhoea; Drug induced liver injury; Fibroid; Heavy menstrual bleeding; Leiomyoma; Levonorgestrel-releasing intrauterine system; Progesterone receptor modulator associated endometrial changes; Quality of life; Randomised controlled trial; Selective progesterone receptor modulator; Ulipristal acetate; Ultrasound; Urgent safety measures; Uterus.
© 2023 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
LHRW, LJM, JPD, LP, SO, VC, CES, MAL, PPS, EPN, NR, SIS and JW all have no conflicts of interest to declare. ARWW has received consultancy fees (divided with the University of Edinburgh) from Bayer AG and Mithra. TJC has received honoraria from Gedeon Richter and Orbis. DKH has received grant funding from Wellbeing of Women, MRC and North West Cancer Research, and honoraria from the Canadian Society of Fertility and Andrology. SB receives grant funding from NHS Grampian Endowments Funding and NIHR, and royalties from Cambridge University Press and payment (to University of Aberdeen) for speaking at 11th Singapore International Congress on Obstetrics & Gynaecology, and invited lectures to Merck, Organon a and Ferring. He participates on the METAFOR Data Monitoring Committee and ANODE trial. He is board member of NHS Grampian for which University of Aberdeen receive payment. He receives an honorium from Oxford University Press for his role as Editor in Chief, (Human Reproduction Open) is special Senior Editor Cochrane Gynaecology and Infertility (no honorarium). LS has received grant funding from NIHR and EU Horizon 2020 and has received consultancy fees from Gideon Richter. RRC has been supported as a Clinical Research Fellow by Bayer AG between April 2018 to February 2021. HODC receives grant funding from Biotechnology and Biological Sciences Research Council, grants from Medical Research Council/NIHR to support salaries for research staff & study consumables and a Research collaboration grant from Bayer AG, Berlin with salaries for research staff & study consumables. She has personal receipt of royalties from "Up-To-Date" for an article on Abnormal Uterine Bleeding. She has received consulting fees to the University of Edinburgh from Bayer AG (Consultancy and Scientific Advisory Board advice; paid to institution), Gedeon Richter (Consultancy advice; paid to institution) and Myovant Sciences GmbH (Consultancy and Scientific Advisory Board advice; paid to institution). She has received speaking fees to the University of Edinburgh from Vifor Pharma UK Ltd for speaking at a meeting on abnormal uterine bleeding and iron deficiency anaemia (paid to institution) and travel expenses from SAB (Scientific Advisory Board) in March 2020. She is Chair (from 2022) of the Committee for Menstrual Disorders and Related Health Impacts of the International Federation of Gynecology and Obstetrics (FIGO; no payment received).
References
-
- National Institute for Health and Care Excellence Heavy menstrual bleeding: assessment and management. https://www.nice.org.uk/guidance/ng88 NICE Guideline, No. 88. - PubMed
-
- Munro M.G., Critchley H.O.D., Fraser I.S., Committee F.M.D. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynaecol Obstet. Dec 2018;143(3):393–408. doi: 10.1002/ijgo.12666. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials