The efficacy of alendronate for the treatment of thalassemia-associated osteoporosis: a randomized controlled trial
- PMID: 37251676
- PMCID: PMC10210588
- DOI: 10.3389/fendo.2023.1178761
The efficacy of alendronate for the treatment of thalassemia-associated osteoporosis: a randomized controlled trial
Abstract
Background: With adequate blood transfusion and iron chelation, thalassemia patients have a longer life expectancy and experience long-term metabolic complications, including osteoporosis, fractures, and bone pain. Alendronate, an oral bisphosphonate, is currently used to treat various types of osteoporosis. However, the efficacy for the treatment of thalassemia-associated osteoporosis remains unclear.
Methods: We conducted a randomized controlled trial to evaluate the efficacy of alendronate for the treatment of osteoporosis in thalassemia patients. Patients were included if they were males (18-50 years) or premenopausal females with low bone mineral density (BMD) (Z-score < -2.0 SD) or positive vertebral deformities from vertebral fracture analysis (VFA). Stratified randomization was performed according to sex and transfusion status. Patients were 1:1 allocated to receive once weekly alendronate 70 mg orally or placebo for a total duration of 12 months. BMD and VFA were re-evaluated at 12 months. Markers of bone resorption (C-terminal crosslinking telopeptide of type I collagen; CTX) and bone formation (Procollagen type I N-terminal propeptide; P1NP), and pain scores were measured at baseline, 6 months, and 12 months. The primary outcome was the change of BMD. The secondary endpoints were changes in bone turnover markers (BTM) and pain scores.
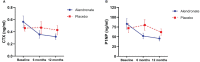
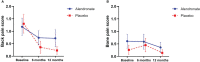
Results: A total of 51 patients received the study drug, 28 patients were assigned to receive alendronate and 23 patients to receive placebo. At 12 months, patients in the alendronate group had significant improvement of BMD at L1-L4 compared to their baseline (0.72 ± 0.11 vs 0.69 ± 0.11 g/cm2, p = 0.004), while there was no change in the placebo group (0.69 ± 0.09 vs 0.70 ± 0.06 g/cm2, p = 0.814). There was no significant change of BMD at femoral neck in both groups. Serum BTMs were significantly decreased among patients receiving alendronate at 6 and 12 months. The mean back pain score was significantly reduced compared to the baseline in both groups (p = 0.003). Side effects were rarely found and led to a discontinuation of the study drug in 1 patient (grade 3 fatigue).
Conclusion: Alendronate 70 mg orally once weekly for 12 months effectively improves BMD at L-spine, reduces serum BTMs, and alleviates back pain in thalassemia patients with osteoporosis. The treatment was well tolerated and had a good safety profile.
Keywords: CTX; P1NP; alendronate; back pain; bisphosphonate; bone mineral density; osteoporosis; thalassemia.
Copyright © 2023 Piriyakhuntorn, Tantiworawit, Phimphilai, Srichairatanakool, Teeyasoontranon, Rattanathammethee, Hantrakool, Chai-Adisaksopha, Rattarittamrong, Norasetthada, Fanhchaksai and Charoenkwan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical