Sphingosine-1-phosphate derived from PRP-Exos promotes angiogenesis in diabetic wound healing via the S1PR1/AKT/FN1 signalling pathway
- PMID: 37251708
- PMCID: PMC10208895
- DOI: 10.1093/burnst/tkad003
Sphingosine-1-phosphate derived from PRP-Exos promotes angiogenesis in diabetic wound healing via the S1PR1/AKT/FN1 signalling pathway
Abstract
Background: Sphingosine-1-phosphate (S1P), a key regulator of vascular homeostasis and angiogenesis, is enriched in exosomes derived from platelet-rich plasma (PRP-Exos). However, the potential role of PRP-Exos-S1P in diabetic wound healing remains unclear. In this study, we investigated the underlying mechanism of PRP-Exos-S1P in diabetic angiogenesis and wound repair.
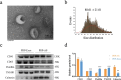
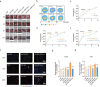
Methods: Exosomes were isolated from PRP by ultracentrifugation and analysed by transmission electron microscopy, nanoparticle tracking analysis and western blotting. The concentration of S1P derived from PRP-Exos was measured by enzyme-linked immunosorbent assay. The expression level of S1P receptor1-3 (S1PR1-3) in diabetic skin was analysed by Q-PCR. Bioinformatics analysis and proteomic sequencing were conducted to explore the possible signalling pathway mediated by PRP-Exos-S1P. A diabetic mouse model was used to evaluate the effect of PRP-Exos on wound healing. Immunofluorescence for cluster of differentiation 31 (CD31) was used to assess angiogenesis in a diabetic wound model.
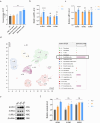
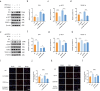
Results: In vitro, PRP-Exos significantly promoted cell proliferation, migration and tube formation. Furthermore, PRP-Exos accelerated the process of diabetic angiogenesis and wound closure in vivo. S1P derived from PRP-Exos was present at a high level, and S1PR1 expression was significantly elevated compared with S1PR2 and S1PR3 in the skin of diabetic patients and animals. However, cell migration and tube formation were not promoted by PRP-Exos-S1P in human umbilical vein endothelial cells treated with shS1PR1. In the diabetic mouse model, inhibition of S1PR1 expression at wounding sites decreased the formation of new blood vessels and delayed the process of wound closure. Bioinformatics analysis and proteomics indicated that fibronectin 1 (FN1) was closely related to S1PR1 due to its colocalization in the endothelial cells of human skin. Further study supported that FN1 plays an important role in the PRP-Exos-S1P-mediated S1PR1/protein kinase B signalling pathway.
Conclusions: PRP-Exos-S1P promotes angiogenesis in diabetic wound healing via the S1PR1/protein kinase B/FN1 signalling pathway. Our findings provide a preliminary theoretical foundation for the treatment of diabetic foot ulcers using PRP-Exos in the future.
Keywords: Diabetic foot ulcer; Exosomes; Platelet-rich plasma; Sphingosine-1-phosphate; Wound healing.
© The Author(s) 2023. Published by Oxford University Press.
Conflict of interest statement
None declared.
Figures




References
-
- Farahani M, Shafiee A. Wound healing: from passive to smart dressings. Adv Healthc Mater. 2021;10:e2100477. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

