Bioconversion of Keratin Wastes Using Keratinolytic Microorganisms to Generate Value-Added Products
- PMID: 37251738
- PMCID: PMC10212687
- DOI: 10.1155/2022/2048031
Bioconversion of Keratin Wastes Using Keratinolytic Microorganisms to Generate Value-Added Products
Abstract
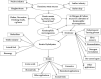
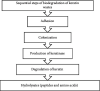
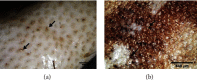
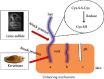
The management of keratinous wastes generated from different industries is becoming a major concern across the world. In each year, more than a billion tons of keratin waste is released into the environment. Despite some trials that have been performed and utilize this waste into valuable products, still a huge amount of keratin waste from different sources is a less explored biomaterial for making valuable products. This indicates that the huge amount of keratin waste is neither disposed properly nor converted into usable products rather thrown away to the environment that causes environmental pollution. Due to the introduction of this waste associated with different pathogenic organisms into soil and water bodies, human beings and other small and large animals are affected by different diseases. Therefore, there is a need for modern and ecofriendly approaches to dispose and convert this waste into usable products. Hence, the objective of this review is to give a concise overview regarding the degradation of keratin waste by biological approaches using keratinase producing microorganisms. The review also focuses on the practical use of keratinases and the economical importance of bioconverted products of keratinous wastes for different applications. Various researches have been studied about the source, disposal mechanisms, techniques of hydrolysis, potential use, and physical and chemical properties of keratin wastes. However, there is negligible information with regard to the use of keratin wastes as media supplements for the growth of keratinolytic microorganisms and silver retrieval from photographic and used X-ray films. Hence, this review differs from other similar reviews in the literature in that it discusses these neglected concerns.
Copyright © 2022 Muhammed Seid Anbesaw.
Conflict of interest statement
The author declares that there are no conflicts of interest.
Figures












Similar articles
-
Comprehensive insights into microbial keratinases and their implication in various biotechnological and industrial sectors: A review.Int J Biol Macromol. 2020 Jul 1;154:567-583. doi: 10.1016/j.ijbiomac.2020.03.116. Epub 2020 Mar 16. Int J Biol Macromol. 2020. PMID: 32194110 Review.
-
Structure, Application, and Biochemistry of Microbial Keratinases.Front Microbiol. 2021 Jun 23;12:674345. doi: 10.3389/fmicb.2021.674345. eCollection 2021. Front Microbiol. 2021. PMID: 34248885 Free PMC article. Review.
-
Valorisation of keratinous wastes: A sustainable approach towards a circular economy.Waste Manag. 2022 Sep;151:81-104. doi: 10.1016/j.wasman.2022.07.021. Epub 2022 Aug 4. Waste Manag. 2022. PMID: 35933837 Review.
-
Harnessing the potential of microbial keratinases for bioconversion of keratin waste.Environ Sci Pollut Res Int. 2024 Oct;31(46):57478-57507. doi: 10.1007/s11356-024-34233-6. Epub 2024 Jul 10. Environ Sci Pollut Res Int. 2024. PMID: 38985428 Review.
-
Sustainable production, biochemical and molecular characterization of thermo-and-solvent stable alkaline serine keratinase from novel Bacillus pumilus AR57 for promising poultry solid waste management.Int J Biol Macromol. 2020 Nov 15;163:135-146. doi: 10.1016/j.ijbiomac.2020.06.219. Epub 2020 Jun 29. Int J Biol Macromol. 2020. PMID: 32615225
Cited by
-
Keratinous bioresources: their generation, microbial degradation, and value enhancement for biotechnological applications.World J Microbiol Biotechnol. 2025 Mar 29;41(4):118. doi: 10.1007/s11274-025-04336-4. World J Microbiol Biotechnol. 2025. PMID: 40155538 Review.
-
Identification of molecular interactions of pesticides with keratinase for their potential to inhibit keratin biodegradation.In Silico Pharmacol. 2024 Jun 8;12(1):54. doi: 10.1007/s40203-024-00229-w. eCollection 2024. In Silico Pharmacol. 2024. PMID: 38860143 Free PMC article.
-
Biodegradation of Keratin Waste by Bacillus velezensis HFS_F2 through Optimized Keratinase Production Medium.Curr Microbiol. 2024 May 18;81(7):179. doi: 10.1007/s00284-024-03699-5. Curr Microbiol. 2024. PMID: 38761211
-
Microbial production of keratinase from Bacillus velezensis strain MAMA: A novel enzyme for eco-friendly degradation of keratin waste.Heliyon. 2024 Jun 4;10(12):e32338. doi: 10.1016/j.heliyon.2024.e32338. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 38988557 Free PMC article.
-
Extraction of Natural-Based Raw Materials Towards the Production of Sustainable Man-Made Organic Fibres.Polymers (Basel). 2024 Dec 23;16(24):3602. doi: 10.3390/polym16243602. Polymers (Basel). 2024. PMID: 39771455 Free PMC article. Review.
References
-
- Edwards D. R., Daniel T. C. Environmental impacts of on-farm poultry waste disposal—a review. Bioresource Technology . 1992;41(1):9–33. doi: 10.1016/0960-8524(92)90094-e. - DOI
-
- Saber W. I. A., El-Metwally M. M., El-Hersh M. S. Keratinase production and biodegradation of some keratinous wastes by Alternaria tenuissima and Aspergillus nidulans. Research Journal of Microbiology . 2010;5(1):21–35. doi: 10.3923/jm.2010.21.35. - DOI
-
- Sharma S., Gupta A. Sustainable management of keratin waste biomass: applications and future perspectives. Brazilian Archives of Biology and Technology . 2016;59:1–14. doi: 10.1590/1678-4324-2016150684. - DOI
-
- Kumawat T. K., Sharma A., Sharma V., Chandra S. The Biodegradable Polymers . London, UK: Intechopen; 2018. Keratin waste; pp. 150–169.
-
- Ritter W. F., Chinside A. E. M. Impact of dead bird disposal pits on groundwater quality on the Delmarva Peninsula. Bioresource Technology . 1995;53(2):105–111. doi: 10.1016/0960-8524(95)00057-l. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

