Biosorption performance of the multi-metal tolerant fungus Aspergillus sp. for removal of some metallic nanoparticles from aqueous solutions
- PMID: 37251841
- PMCID: PMC10209406
- DOI: 10.1016/j.heliyon.2023.e16125
Biosorption performance of the multi-metal tolerant fungus Aspergillus sp. for removal of some metallic nanoparticles from aqueous solutions
Abstract
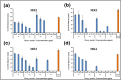
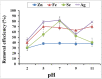
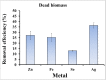
The wide spread of nanotechnology applications currently carries with it the possibility of polluting the environment with the residues of these nanomaterials, especially those in the metallic form. Therefore, it is necessary to study the possibility of treating and removing various nanoscale metal pollutants in environmentally friendly ways. The present study focused on the isolation of multi-metal tolerant fungi to be applied in the bioremoval of Zn, Fe, Se, and Ag nanoparticles as potential nanoscale metal pollutants. Aspergillus sp. has been isolated as multi-metal tolerant fingus and investigated in the bioremoval of targeted nanometals from their aquoues solutions. The effect of biomass age, pH, and contact time was studied to determine the optimal biosorption conditions for fungal pellets towards metal NPs. The results showed a high percentage of fungal biosorption on the of two-day-old cells, which amounted to 39.3, 52.2, 91.7, and 76.8% of zinc, iron, selenium, and silver, respectively. The pH 7 was recorded the highest percentage of NPs removal for the four studied metals i.e. 38.8, 68.1, 80.4, and 82.0% of Zn-, Fe-, Se- and Ag-NPs, respectively. The contact time required between Aspergillus sp. and the metal nanoparticles to obtain the best adsorption was only 10 min in the case of Zn and Ag, but it was 40 min for both Fe and Se NPs. The efficiency of living fungal pellets in removing the four metallic NPs exceeded that of dead biomass by 1.8, 5.7, 2.5, and 2.5 folds for Zn, Fe, Se and Ag, respectively. However, utilization of dead fungal biomass for metallic NPs removal could be considered more applicable to the actual environmental applications.
Keywords: Aspergillus sp.; Bioremediation; Biosorption; Metallic nanoparticles.
© 2023 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Hofman-Caris C.H.M., Bäuerlein P.S., Siegers W.G., Mintenig S.M., Messina R., Dekker S.C., Bertelkamp Ch, Cornelissen E.R., van Wezel A.P. Removal of nanoparticles (both inorganic nanoparticles and nanoplastics) in drinking water treatment - coagulation/flocculation/sedimentation, and sand/granular activated carbon filtration. Environ. Sci.: Water Res. Technol. 2022;8:1675–1686. doi: 10.1039/d2ew00226d. - DOI
-
- Saleh H.A., Matter I.A., Abdel‐Wareth M.T.A., Darwesh O.M. Molluscicidal, histopathological and genotoxic effects of Scenedesmus obliquus and Spirulina platensis extracts and their biosynthesized zinc oxide nanoparticles on Biomphalaria alexandrina snails. Aquacult. Res. 2022;53(10):3680–3695. doi: 10.1111/are.15872. - DOI
-
- Darwesh O.M., Ali S.S., Matter I.A., Elsamahy T. In: Nanosensors and Nanodevices for Smart Multifunctional Textiles. Andrea E., Nguyen T.A., Nguyen-Tri P., editors. Elsevier; 2021. Nanotextiles waste management: controlling of release and remediation of wastes; pp. 267–286. - DOI
LinkOut - more resources
Full Text Sources

