Alzheimer's disease drug development pipeline: 2023
- PMID: 37251912
- PMCID: PMC10210334
- DOI: 10.1002/trc2.12385
Alzheimer's disease drug development pipeline: 2023
Erratum in
-
Erratum: Alzheimer's disease drug development pipeline: 2023.Alzheimers Dement (N Y). 2023 Jun 28;9(2):e12407. doi: 10.1002/trc2.12407. eCollection 2023 Apr-Jun. Alzheimers Dement (N Y). 2023. PMID: 37388760 Free PMC article.
Abstract
Introduction: Drugs that prevent the onset, slow progression, or improve cognitive and behavioral symptoms of Alzheimer's disease (AD) are needed.
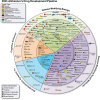
Methods: We searched ClinicalTrials.gov for all current Phase 1, 2 and 3 clinical trials for AD and mild cognitive impairment (MCI) attributed to AD. We created an automated computational database platform to search, archive, organize, and analyze the derived data. The Common Alzheimer's Disease Research Ontology (CADRO) was used to identify treatment targets and drug mechanisms.
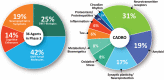
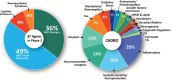
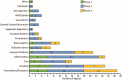
Results: On the index date of January 1, 2023, there were 187 trials assessing 141 unique treatments for AD. Phase 3 included 36 agents in 55 trials; 87 agents were in 99 Phase 2 trials; and Phase 1 had 31 agents in 33 trials. Disease-modifying therapies were the most common drugs comprising 79% of drugs in trials. Twenty-eight percent of candidate therapies are repurposed agents. Populating all current Phase 1, 2, and 3 trials will require 57,465 participants.
Discussion: The AD drug development pipeline is advancing agents directed at a variety of target processes.
Highlights: There are currently 187 trials assessing 141 drugs for the treatment of Alzheimer's disease (AD).Drugs in the AD pipeline address a variety of pathological processes.More than 57,000 participants will be required to populate all currently registered trials.
Keywords: Alzheimer's disease; Common Alzheimer's Disease Research Ontology (CADRO); amyloid; biomarkers; clinical trials; drug development; inflammation; pharmaceutical companies; repurposed drugs; synaptic function; tau.
© 2023 The Authors. Alzheimer's & Dementia: Translational Research & Clinical Interventions published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
J.C. has provided consultation to Acadia, Alkahest, AlphaCognition, AriBio, Avanir, Axsome, Behren Therapeutics, Biogen, Biohaven, Cassava, Cerecin, Cortexyme, Diadem, EIP Pharma, Eisai, GemVax, Genentech, Green Valley, Grifols, Janssen, LSP, Merck, NervGen, Novo Nordisk, Oligomerix, Ono, Otsuka, PRODEO, ReMYND, Renew, Resverlogix, Roche, Signant Health, Suven, United Neuroscience, and Unlearn AI pharmaceutical, assessment, and investment companies. J.C. is supported by NIGMS grant P20GM109025. NINDS grant U01NS093334. NIA grant R01AG053798. NIA grant P20AG068053. NIA grant R35AG71476. and the Alzheimer's Disease Drug Discovery Foundation (ADDF). J.C. owns the copyright of the Neuropsychiatric Inventory. G.L. is a full‐time employee of Biogen. K.Z. is the CEO of CNS Innovations. Y.Z. and J.F. declare no competing interests. F.C. is supported by the NIA under Award Numbers U01AG073323, R01AG076448, R01AG082211, R01AG066707, 3R01AG066707‐01S1, 3R01AG066707‐02S1, R56AG074001, and R35AG71476. F.C. declares no other competing interests. Author disclosures are available in thesupporting information.
Figures
References
-
- Alzheimer's Association . 2021 Alzheimer's disease facts and figures. Alzheimers Dement. 2021;17:327‐406. - PubMed
-
- Gustavsson A, Norton N, Fast T, et al. Global estimates on the number of persons across the Alzheimer's disease continuum. Alzheimers Dement. 2023; 19(2):658‐670. - PubMed
-
- Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer's disease. Lancet. 2021;S0140‐6736(20):32205‐32204.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
