Highly efficient cell-microbead encapsulation using dielectrophoresis-assisted dual-nanowell array
- PMID: 37252002
- PMCID: PMC10210622
- DOI: 10.1093/pnasnexus/pgad155
Highly efficient cell-microbead encapsulation using dielectrophoresis-assisted dual-nanowell array
Abstract
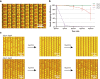
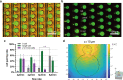
Recent advancements in micro/nanofabrication techniques have led to the development of portable devices for high-throughput single-cell analysis through the isolation of individual target cells, which are then paired with functionalized microbeads. Compared with commercially available benchtop instruments, portable microfluidic devices can be more widely and cost-effectively adopted in single-cell transcriptome and proteome analysis. The sample utilization and cell pairing rate (∼33%) of current stochastic-based cell-bead pairing approaches are fundamentally limited by Poisson statistics. Despite versatile technologies having been proposed to reduce randomness during the cell-bead pairing process in order to statistically beat the Poisson limit, improvement of the overall pairing rate of a single cell to a single bead is typically based on increased operational complexity and extra instability. In this article, we present a dielectrophoresis (DEP)-assisted dual-nanowell array (ddNA) device, which employs an innovative microstructure design and operating process that decouples the bead- and cell-loading processes. Our ddNA design contains thousands of subnanoliter microwell pairs specifically tailored to fit both beads and cells. Interdigitated electrodes (IDEs) are placed below the microwell structure to introduce a DEP force on cells, yielding high single-cell capture and pairing rates. Experimental results with human embryonic kidney cells confirmed the suitability and reproducibility of our design. We achieved a single-bead capture rate of >97% and a cell-bead pairing rate of >75%. We anticipate that our device will enhance the application of single-cell analysis in practical clinical use and academic research.
Keywords: co-encapsulation; dielectrophoresis; hydrophilic beads; microfluidics; single-cell capture.
© The Author(s) 2023. Published by Oxford University Press on behalf of National Academy of Sciences.
Figures



References
LinkOut - more resources
Full Text Sources

